Pertuzumab and Trastuzumab Combination with Concomitant Locoregional Radiotherapy for the Treatment of Breast Cancers with HER2 Receptor Overexpression
- PMID: 34638275
- PMCID: PMC8507738
- DOI: 10.3390/cancers13194790
Pertuzumab and Trastuzumab Combination with Concomitant Locoregional Radiotherapy for the Treatment of Breast Cancers with HER2 Receptor Overexpression
Abstract
Background: The combination of pertuzumab and trastuzumab dual HER2 blockade with concomitant curative dose locoregional breast radiotherapy in patients with metastatic breast cancer is an important part of treatment strategy.
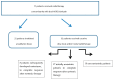
Methods: This was a retrospective study conducted at the Institut Curie on all patients treated concomitantly with pertuzumab/trastuzumab and locoregional breast radiotherapy. Toxicity was evaluated according to the NCICTCAEv4.0. Overall survival, progression-free survival and locoregional recurrence-free survival were evaluated in metastatic patients who were initially well controlled by chemotherapy, for whom local treatment was decided by the multidisciplinary team.
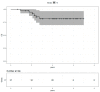
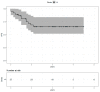
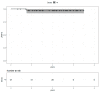
Results: Fifty-five patients treated between October 2013 and December 2019 were included, with a median follow-up of 4.1 years. The median age was 53 years (range: 28-81). All patients received curative dose radiotherapy (RT) concomitantly with pertuzumab and trastuzumab (Pertu/Trastu). The median radiation dose was 50 Gy. Safety evaluation did not reveal any significant adverse effects, with 3 cases of grade 3 radiodermatitis (5.4%), but no significant gastrointestinal or cardiac toxicity. The mean difference in LVEF before any chemotherapy and after radiotherapy was -2.43% (p < 0.01).
Conclusions: This study demonstrates that the combination of locoregional breast RT with dual HER2 blockade by Pertu/Trastu was very well tolerated, suggesting that RT can be safely administered to patients with HER2-positive breast cancer.
Keywords: breast cancer; concurrent pertuzumab-trastuzumab-radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Concurrent radiation therapy and dual HER2 blockade in breast cancer: Assessment of toxicity].Cancer Radiother. 2021 Jul;25(5):424-431. doi: 10.1016/j.canrad.2020.06.037. Epub 2021 Mar 24. Cancer Radiother. 2021. PMID: 33771453 French.
-
Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): an open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group.Lancet Oncol. 2018 Mar;19(3):323-336. doi: 10.1016/S1470-2045(18)30083-4. Epub 2018 Feb 9. Lancet Oncol. 2018. PMID: 29433963 Clinical Trial.
-
Combination of radiotherapy and double blockade HER2 with pertuzumab and trastuzumab for HER2-positive metastatic or locally recurrent unresectable and/or metastatic breast cancer: Assessment of early toxicity.Cancer Radiother. 2017 Apr;21(2):114-118. doi: 10.1016/j.canrad.2016.10.002. Epub 2017 Mar 24. Cancer Radiother. 2017. PMID: 28347625
-
Pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer.Expert Rev Anticancer Ther. 2015 Jan;15(1):17-26. doi: 10.1586/14737140.2015.992418. Epub 2014 Dec 12. Expert Rev Anticancer Ther. 2015. PMID: 25494663 Review.
-
Pertuzumab for the treatment of human epidermal growth factor receptor type 2-positive metastatic breast cancer.Am J Health Syst Pharm. 2013 Sep 15;70(18):1579-87. doi: 10.2146/ajhp120735. Am J Health Syst Pharm. 2013. PMID: 23988598 Review.
Cited by
-
Proton therapy reduces the effective dose to immune cells in breast cancer patients.Strahlenther Onkol. 2024 Dec;200(12):1074-1079. doi: 10.1007/s00066-024-02263-1. Epub 2024 Jul 25. Strahlenther Onkol. 2024. PMID: 39060636
-
Determinants of radiation dose to immune cells during breast radiotherapy.Strahlenther Onkol. 2024 May 27. doi: 10.1007/s00066-024-02240-8. Online ahead of print. Strahlenther Onkol. 2024. PMID: 38801448
-
Interaction between Radiation Therapy and Targeted Therapies in HER2-Positive Breast Cancer: Literature Review, Levels of Evidence for Safety and Recommendations for Optimal Treatment Sequence.Cancers (Basel). 2023 Apr 13;15(8):2278. doi: 10.3390/cancers15082278. Cancers (Basel). 2023. PMID: 37190205 Free PMC article. Review.
-
Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers.Open Life Sci. 2023 Jan 10;18(1):20220535. doi: 10.1515/biol-2022-0535. eCollection 2023. Open Life Sci. 2023. PMID: 36694697 Free PMC article.
-
HLX11, a Proposed Pertuzumab Biosimilar: Pharmacokinetics, Immunogenicity, and Safety Profiles Compared to Three Reference Biologic Products (US-, EU-, and CN-Approved Pertuzumab) Administered to Healthy Male Subjects.BioDrugs. 2022 May;36(3):393-409. doi: 10.1007/s40259-022-00534-w. Epub 2022 May 20. BioDrugs. 2022. PMID: 35594017 Free PMC article. Clinical Trial.
References
-
- Barok M., Isola J., Pályi-Krekk Z., Nagy P., Juhász I., Vereb G., Kauraniemi P., Kapanen A., Tanner M., Szöllösi J. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol. Cancer Ther. 2007;6:2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous


