Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates
- PMID: 33053279
- PMCID: PMC7583697
- DOI: 10.1056/NEJMoa2027906
Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and the resulting disease, coronavirus disease 2019 (Covid-19), have spread to millions of persons worldwide. Multiple vaccine candidates are under development, but no vaccine is currently available. Interim safety and immunogenicity data about the vaccine candidate BNT162b1 in younger adults have been reported previously from trials in Germany and the United States.
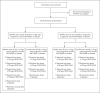
Methods: In an ongoing, placebo-controlled, observer-blinded, dose-escalation, phase 1 trial conducted in the United States, we randomly assigned healthy adults 18 to 55 years of age and those 65 to 85 years of age to receive either placebo or one of two lipid nanoparticle-formulated, nucleoside-modified RNA vaccine candidates: BNT162b1, which encodes a secreted trimerized SARS-CoV-2 receptor-binding domain; or BNT162b2, which encodes a membrane-anchored SARS-CoV-2 full-length spike, stabilized in the prefusion conformation. The primary outcome was safety (e.g., local and systemic reactions and adverse events); immunogenicity was a secondary outcome. Trial groups were defined according to vaccine candidate, age of the participants, and vaccine dose level (10 μg, 20 μg, 30 μg, and 100 μg). In all groups but one, participants received two doses, with a 21-day interval between doses; in one group (100 μg of BNT162b1), participants received one dose.
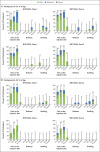
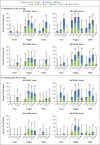
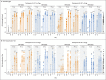
Results: A total of 195 participants underwent randomization. In each of 13 groups of 15 participants, 12 participants received vaccine and 3 received placebo. BNT162b2 was associated with a lower incidence and severity of systemic reactions than BNT162b1, particularly in older adults. In both younger and older adults, the two vaccine candidates elicited similar dose-dependent SARS-CoV-2-neutralizing geometric mean titers, which were similar to or higher than the geometric mean titer of a panel of SARS-CoV-2 convalescent serum samples.
Conclusions: The safety and immunogenicity data from this U.S. phase 1 trial of two vaccine candidates in younger and older adults, added to earlier interim safety and immunogenicity data regarding BNT162b1 in younger adults from trials in Germany and the United States, support the selection of BNT162b2 for advancement to a pivotal phase 2-3 safety and efficacy evaluation. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04368728.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Update of
-
RNA-Based COVID-19 Vaccine BNT162b2 Selected for a Pivotal Efficacy Study.medRxiv [Preprint]. 2020 Aug 28:2020.08.17.20176651. doi: 10.1101/2020.08.17.20176651. medRxiv. 2020. Update in: N Engl J Med. 2020 Dec 17;383(25):2439-2450. doi: 10.1056/NEJMoa2027906. PMID: 32839784 Free PMC article. Updated. Preprint.
Comment in
-
Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated With Belatacept.Transplantation. 2021 Sep 1;105(9):e94-e95. doi: 10.1097/TP.0000000000003784. Transplantation. 2021. PMID: 33831941 No abstract available.
Similar articles
-
Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study.Nat Med. 2021 Jun;27(6):1062-1070. doi: 10.1038/s41591-021-01330-9. Epub 2021 Apr 22. Nat Med. 2021. PMID: 33888900 Clinical Trial.
-
Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults.N Engl J Med. 2020 Dec 17;383(25):2427-2438. doi: 10.1056/NEJMoa2028436. Epub 2020 Sep 29. N Engl J Med. 2020. PMID: 32991794 Free PMC article. Clinical Trial.
-
RNA-Based COVID-19 Vaccine BNT162b2 Selected for a Pivotal Efficacy Study.medRxiv [Preprint]. 2020 Aug 28:2020.08.17.20176651. doi: 10.1101/2020.08.17.20176651. medRxiv. 2020. Update in: N Engl J Med. 2020 Dec 17;383(25):2439-2450. doi: 10.1056/NEJMoa2027906. PMID: 32839784 Free PMC article. Updated. Preprint.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Tozinameran (BNT162b2) Vaccine: The Journey from Preclinical Research to Clinical Trials and Authorization.AAPS PharmSciTech. 2021 Jun 7;22(5):172. doi: 10.1208/s12249-021-02058-y. AAPS PharmSciTech. 2021. PMID: 34100150 Free PMC article. Review.
Cited by
-
The BNT162b2 mRNA vaccine demonstrates reduced age-associated TH1 support in vitro and in vivo.iScience. 2024 Sep 26;27(11):111055. doi: 10.1016/j.isci.2024.111055. eCollection 2024 Nov 15. iScience. 2024. PMID: 39569372 Free PMC article.
-
Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications.Signal Transduct Target Ther. 2024 Nov 14;9(1):322. doi: 10.1038/s41392-024-02002-z. Signal Transduct Target Ther. 2024. PMID: 39543114 Free PMC article. Review.
-
Nano-enhanced immunity: A bibliometric analysis of nanoparticles in vaccine adjuvant research.Hum Vaccin Immunother. 2024 Dec 31;20(1):2427464. doi: 10.1080/21645515.2024.2427464. Epub 2024 Nov 14. Hum Vaccin Immunother. 2024. PMID: 39539151 Free PMC article. Review.
-
Rationally designed multimeric nanovaccines using icosahedral DNA origami for display of SARS-CoV-2 receptor binding domain.Nat Commun. 2024 Nov 6;15(1):9581. doi: 10.1038/s41467-024-53937-4. Nat Commun. 2024. PMID: 39505890 Free PMC article.
-
Engineering a SARS-CoV-2 Vaccine Targeting the Receptor-Binding Domain Cryptic-Face via Immunofocusing.ACS Cent Sci. 2024 Sep 17;10(10):1871-1884. doi: 10.1021/acscentsci.4c00722. eCollection 2024 Oct 23. ACS Cent Sci. 2024. PMID: 39463836 Free PMC article.
References
-
- Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020 (https://coronavirus.jhu.edu/map.html).
-
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020. September 30 (Epub ahead of print). - PubMed
-
- Mulligan MJ, Lyke KE, Kitchin N, et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020. August 12 (Epub ahead of print). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

