Long-term safety and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy
- PMID: 31755090
- PMCID: PMC6988520
- DOI: 10.1111/epi.16381
Long-term safety and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy
Erratum in
-
Corrigendum.Epilepsia. 2021 Apr;62(4):1039. doi: 10.1111/epi.16767. Epub 2020 Nov 25. Epilepsia. 2021. PMID: 33836101 Free PMC article. No abstract available.
Abstract
Objective: A large-scale, double-blind trial (SP0993; NCT01243177) demonstrated that lacosamide was noninferior to controlled-release carbamazepine (carbamazepine-CR) in terms of efficacy, and well tolerated as first-line monotherapy in patients (≥16 years of age) with newly diagnosed epilepsy. We report primary safety outcomes from the double-blind extension of the noninferiority trial (SP0994; NCT01465997) and post hoc analyses of pooled long-term safety and efficacy data from both trials.
Methods: Patients were randomized 1:1 to lacosamide or carbamazepine-CR. Doses were escalated (lacosamide: 200/400/600 mg/d; carbamazepine-CR: 400/800/1200 mg/d) based on seizure control. Eligible patients continued randomized treatment in the extension. Primary outcomes of the extension were treatment-emergent adverse events (TEAEs), serious TEAEs, and discontinuations due to TEAEs. Post hoc analyses of data from combined trials included 12- and 24-month seizure freedom and TEAEs by number of comorbid conditions.
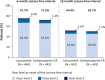
Results: A total of 886 patients were treated in the initial trial and 548 in the extension; 211 of 279 patients (75.6%) on lacosamide and 180/269 (66.9%) on carbamazepine-CR completed the extension. In the extension, 181 patients (64.9%) on lacosamide and 182 (67.7%) on carbamazepine-CR reported TEAEs; in both groups, nasopharyngitis, headache, and dizziness were most common. Serious TEAEs were reported by 32 patients (11.5%) on lacosamide and 22 (8.2%) on carbamazepine-CR; 12 (4.3%) and 21 (7.8%) discontinued due to TEAEs. In the combined trials (median exposure: lacosamide 630 days; carbamazepine-CR 589 days), Kaplan-Meier estimated proportions of patients with 12- and 24-month seizure freedom from first dose were 50.8% (95% confidence interval 46.2%-55.4%) and 47.0% (42.2%-51.7%) on lacosamide, and 54.9% (50.3%-59.6%) and 50.9% (46.0%-55.7%) on carbamazepine-CR. Incidences of drug-related TEAEs and discontinuations due to TEAEs increased by number of comorbid conditions and were lower in patients on lacosamide.
Significance: Long-term (median ~2 years) lacosamide monotherapy was efficacious and generally well tolerated in adults with newly diagnosed epilepsy. Seizure freedom rates were similar with lacosamide and carbamazepine-CR.
Keywords: antiepileptic drug; comorbidity; lacosamide monotherapy; tolerability.
© 2019 UCB Biopharma SPRL. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Conflict of interest statement
Elinor Ben‐Menachem has served as a paid consultant for Eisai, Sandoz, and UCB Pharma; has received research grants from Eisai, GW Pharmaceuticals, SK Life Science, and UCB Pharma; and is the Editor‐in‐Chief of
Figures

Similar articles
-
Tolerability and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy and concomitant psychiatric conditions: Post hoc analysis of a prospective, randomized, double-blind trial.Epilepsy Res. 2020 Jan;159:106220. doi: 10.1016/j.eplepsyres.2019.106220. Epub 2019 Oct 16. Epilepsy Res. 2020. PMID: 31812127 Clinical Trial.
-
Efficacy and tolerability of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed temporal lobe epilepsy: Post hoc analysis of a randomized, double-blind trial.Seizure. 2023 Nov;112:62-67. doi: 10.1016/j.seizure.2023.09.011. Epub 2023 Sep 13. Seizure. 2023. PMID: 37769545 Clinical Trial.
-
Lacosamide in patients with epilepsy of cerebrovascular etiology.Acta Neurol Scand. 2020 Jun;141(6):473-482. doi: 10.1111/ane.13230. Epub 2020 Mar 13. Acta Neurol Scand. 2020. PMID: 32068241 Clinical Trial.
-
Immediate-release versus controlled-release carbamazepine in the treatment of epilepsy.Cochrane Database Syst Rev. 2014 Feb 3;(2):CD007124. doi: 10.1002/14651858.CD007124.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2014 Dec 03;(12):CD007124. doi: 10.1002/14651858.CD007124.pub4 PMID: 24488654 Updated. Review.
-
Immediate-release versus controlled-release carbamazepine in the treatment of epilepsy.Cochrane Database Syst Rev. 2010 Jan 20;(1):CD007124. doi: 10.1002/14651858.CD007124.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2014 Feb 03;(2):CD007124. doi: 10.1002/14651858.CD007124.pub3 PMID: 20091617 Updated. Review.
Cited by
-
Lacosamide as an Adjunctive Therapy in Drug-Resistant Absence Epilepsy: Successful Treatment of Four Patients.Iran J Child Neurol. 2024 Fall;18(4):121-126. doi: 10.22037/ijcn.v18i4.45400. Epub 2024 Sep 29. Iran J Child Neurol. 2024. PMID: 39478942
-
Switching carbamazepine to lacosamide improves gamma-glutamyl transpeptidase levels.Epilepsia Open. 2024 Oct;9(5):1956-1961. doi: 10.1002/epi4.13018. Epub 2024 Aug 14. Epilepsia Open. 2024. PMID: 39141546 Free PMC article.
-
Efficacy, safety, and tolerability of adjunctive Lacosamide therapy for focal seizures in young children aged ≥1 month to ≤4 years: A real-world study.CNS Neurosci Ther. 2024 Aug;30(8):e14917. doi: 10.1111/cns.14917. CNS Neurosci Ther. 2024. PMID: 39123302 Free PMC article.
-
Risk assessment of arrhythmias related to three antiseizure medications: a systematic review and single-arm meta-analysis.Front Neurol. 2024 Feb 14;15:1295368. doi: 10.3389/fneur.2024.1295368. eCollection 2024. Front Neurol. 2024. PMID: 38419702 Free PMC article.
-
Safety and efficacy of levetiracetam and carbamazepine monotherapy in the management of pediatric focal epilepsy: a randomized clinical trial.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):5233-5240. doi: 10.1007/s00210-024-02954-7. Epub 2024 Jan 24. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38265679 Clinical Trial.
References
-
- Vimpat® (lacosamide) Summary of Product Characteristics. Brussels, Belgium: UCB Pharma, SA; 2018. Available at: https://www.ema.europa.eu/documents/product-information/vimpat-epar-prod.... Accessed June 10, 2019.
-
- Vimpat® (lacosamide) C‐V Prescribing Information. Smyrna, GA: UCB, Inc; 2019. Available at: https://www.vimpat.com/vimpat-prescribing-information.pdf. Accessed June 10, 2019.
-
- Ben‐Menachem E, Biton V, Jatuzis D, Abou‐Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial‐onset seizures. Epilepsia. 2007;48:1308–17. - PubMed
-
- Halász P, Kälviäinen R, Mazurkiewicz‐Beldzińska M, Rosenow F, Doty P, Hebert D, et al. Adjunctive lacosamide for partial‐onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50:443–53. - PubMed


