The Long Noncoding RNA MALAT1 Induces Tolerogenic Dendritic Cells and Regulatory T Cells via miR155/Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin/IL10 Axis
- PMID: 30150986
- PMCID: PMC6099154
- DOI: 10.3389/fimmu.2018.01847
The Long Noncoding RNA MALAT1 Induces Tolerogenic Dendritic Cells and Regulatory T Cells via miR155/Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin/IL10 Axis
Erratum in
-
Corrigendum: The Long Noncoding RNA MALAT1 Induces Tolerogenic Dendritic Cells and Regulatory T Cells via miR155/Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin/IL10 Axis.Front Immunol. 2020 Oct 16;11:582491. doi: 10.3389/fimmu.2020.582491. eCollection 2020. Front Immunol. 2020. PMID: 33178211 Free PMC article.
Abstract
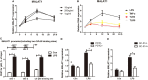
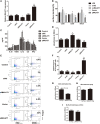
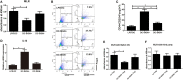
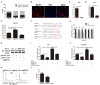
By shaping T cell immunity, tolerogenic dendritic cells (tDCs) play critical roles in the induction of immune tolerance after transplantation. However, the role of long noncoding RNAs (lncRNAs) in the function and immune tolerance of dendritic cells (DCs) is largely unknown. Here, we found that the lncRNA MALAT1 is upregulated in the infiltrating cells of tolerized mice with cardiac allografts and activated DCs. Functionally, MALAT1 overexpression favored a switch in DCs toward a tolerant phenotype. Mechanistically, ectopic MALAT1 promoted dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN) expression by functioning as an miR155 sponge, which is essential for the tolerogenic maintenance of DCs and the DC-SIGN-positive subset with more potent tolerogenic ability. The adoptive transfer of MALAT1-overexpressing DCs promoted cardiac allograft survival and protected from the development of experimental autoimmune myocarditis, accompanied with increasing antigen-specific regulatory T cells. Therefore, overexpressed MALAT1 induces tDCs and immune tolerance in heart transplantation and autoimmune disease by the miRNA-155/DC-SIGH/IL10 axis. This study highlights that the lncRNA MALAT1 is a novel tolerance regulator in immunity that has important implications in settings in which tDCs are preferred.
Keywords: IL10; MALAT1 long noncoding RNA; dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin; immune tolerance; miR155; tolerogenic dendritic cell.
Figures


Similar articles
-
Corrigendum: The Long Noncoding RNA MALAT1 Induces Tolerogenic Dendritic Cells and Regulatory T Cells via miR155/Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin/IL10 Axis.Front Immunol. 2020 Oct 16;11:582491. doi: 10.3389/fimmu.2020.582491. eCollection 2020. Front Immunol. 2020. PMID: 33178211 Free PMC article.
-
Knockdown of NEAT1 induces tolerogenic phenotype in dendritic cells by inhibiting activation of NLRP3 inflammasome.Theranostics. 2019 May 24;9(12):3425-3442. doi: 10.7150/thno.33178. eCollection 2019. Theranostics. 2019. PMID: 31281488 Free PMC article.
-
Myosin-primed tolerogenic dendritic cells ameliorate experimental autoimmune myocarditis.Cardiovasc Res. 2014 Feb 1;101(2):203-10. doi: 10.1093/cvr/cvt246. Epub 2013 Nov 4. Cardiovasc Res. 2014. PMID: 24189626
-
Regulatory dendritic cells in autoimmunity: A comprehensive review.J Autoimmun. 2015 Sep;63:1-12. doi: 10.1016/j.jaut.2015.07.011. Epub 2015 Aug 5. J Autoimmun. 2015. PMID: 26255250 Review.
-
DC-SIGN and LFA-1: a battle for ligand.Trends Immunol. 2001 Aug;22(8):457-63. doi: 10.1016/s1471-4906(01)01974-3. Trends Immunol. 2001. PMID: 11473836 Review.
Cited by
-
Non-Coding RNAs in Myasthenia Gravis: From Immune Regulation to Personalized Medicine.Cells. 2024 Sep 14;13(18):1550. doi: 10.3390/cells13181550. Cells. 2024. PMID: 39329732 Free PMC article. Review.
-
Biological functions and affected signaling pathways by Long Non-Coding RNAs in the immune system.Noncoding RNA Res. 2024 Sep 6;10:70-90. doi: 10.1016/j.ncrna.2024.09.001. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39315339 Free PMC article. Review.
-
LncRNAs in Immune and Stromal Cells Remodel Phenotype of Cancer Cell and Tumor Microenvironment.J Inflamm Res. 2024 May 17;17:3173-3185. doi: 10.2147/JIR.S460730. eCollection 2024. J Inflamm Res. 2024. PMID: 38774447 Free PMC article. Review.
-
Pivotal functions and impact of long con-coding RNAs on cellular processes and genome integrity.J Biomed Sci. 2024 May 14;31(1):52. doi: 10.1186/s12929-024-01038-1. J Biomed Sci. 2024. PMID: 38745221 Free PMC article. Review.
-
The significance of long non-coding RNAs in the pathogenesis, diagnosis and treatment of inflammatory bowel disease.Precis Clin Med. 2023 Dec 11;6(4):pbad031. doi: 10.1093/pcmedi/pbad031. eCollection 2023 Dec. Precis Clin Med. 2023. PMID: 38163004 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


