Mathematical Modeling of Protein Misfolding Mechanisms in Neurological Diseases: A Historical Overview
- PMID: 29456521
- PMCID: PMC5801313
- DOI: 10.3389/fneur.2018.00037
Mathematical Modeling of Protein Misfolding Mechanisms in Neurological Diseases: A Historical Overview
Abstract
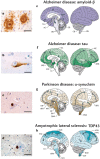
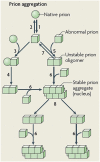
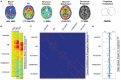
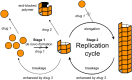
Protein misfolding refers to a process where proteins become structurally abnormal and lose their specific 3-dimensional spatial configuration. The histopathological presence of misfolded protein (MP) aggregates has been associated as the primary evidence of multiple neurological diseases, including Prion diseases, Alzheimer's disease, Parkinson's disease, and Creutzfeldt-Jacob disease. However, the exact mechanisms of MP aggregation and propagation, as well as their impact in the long-term patient's clinical condition are still not well understood. With this aim, a variety of mathematical models has been proposed for a better insight into the kinetic rate laws that govern the microscopic processes of protein aggregation. Complementary, another class of large-scale models rely on modern molecular imaging techniques for describing the phenomenological effects of MP propagation over the whole brain. Unfortunately, those neuroimaging-based studies do not take full advantage of the tremendous capabilities offered by the chemical kinetics modeling approach. Actually, it has been barely acknowledged that the vast majority of large-scale models have foundations on previous mathematical approaches that describe the chemical kinetics of protein replication and propagation. The purpose of the current manuscript is to present a historical review about the development of mathematical models for describing both microscopic processes that occur during the MP aggregation and large-scale events that characterize the progression of neurodegenerative MP-mediated diseases.
Keywords: mathematical modeling; misfolded protein; neurodegeneration; prion-like hypothesis; therapeutic interventions.
Figures
Similar articles
-
Diverse Misfolded Conformational Strains and Cross-seeding of Misfolded Proteins Implicated in Neurodegenerative Diseases.Front Mol Neurosci. 2019 Jul 9;12:158. doi: 10.3389/fnmol.2019.00158. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31338019 Free PMC article.
-
How an Infection of Sheep Revealed Prion Mechanisms in Alzheimer's Disease and Other Neurodegenerative Disorders.Int J Mol Sci. 2021 May 4;22(9):4861. doi: 10.3390/ijms22094861. Int J Mol Sci. 2021. PMID: 34064393 Free PMC article. Review.
-
[Can prion-like propagation occur in neurodegenerative diseases?: in view of transmissible systemic amyloidosis].Brain Nerve. 2012 Jun;64(6):665-74. Brain Nerve. 2012. PMID: 22647474 Review. Japanese.
-
Generalization of the prion hypothesis to other neurodegenerative diseases: an imperfect fit.J Toxicol Environ Health A. 2011;74(22-24):1433-59. doi: 10.1080/15287394.2011.618967. J Toxicol Environ Health A. 2011. PMID: 22043906 Review.
-
Prion Diseases: A Unique Transmissible Agent or a Model for Neurodegenerative Diseases?Biomolecules. 2021 Feb 2;11(2):207. doi: 10.3390/biom11020207. Biomolecules. 2021. PMID: 33540845 Free PMC article. Review.
Cited by
-
Beyond the usual suspects: multi-factorial computational models in the search for neurodegenerative disease mechanisms.Transl Psychiatry. 2024 Sep 23;14(1):386. doi: 10.1038/s41398-024-03073-w. Transl Psychiatry. 2024. PMID: 39313512 Free PMC article. Review.
-
Exosomes in neuron-glia communication: A review on neurodegeneration.Bioimpacts. 2024;14(5):30153. doi: 10.34172/bi.2023.30153. Epub 2024 Jan 7. Bioimpacts. 2024. PMID: 39296798 Free PMC article. Review.
-
In-vivo neuronal dysfunction by Aβ and tau overlaps with brain-wide inflammatory mechanisms in Alzheimer's disease.Front Aging Neurosci. 2024 Jun 19;16:1383163. doi: 10.3389/fnagi.2024.1383163. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38966801 Free PMC article.
-
A scoping review of mathematical models covering Alzheimer's disease progression.Front Neuroinform. 2024 Mar 14;18:1281656. doi: 10.3389/fninf.2024.1281656. eCollection 2024. Front Neuroinform. 2024. PMID: 38550514 Free PMC article. Review.
-
A Meta-Analysis Study of SOD1-Mutant Mouse Models of ALS to Analyse the Determinants of Disease Onset and Progression.Int J Mol Sci. 2022 Dec 22;24(1):216. doi: 10.3390/ijms24010216. Int J Mol Sci. 2022. PMID: 36613659 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources


