Evaluating dengue burden in Africa in passive fever surveillance and seroprevalence studies: protocol of field studies of the Dengue Vaccine Initiative
- PMID: 29358421
- PMCID: PMC5780679
- DOI: 10.1136/bmjopen-2017-017673
Evaluating dengue burden in Africa in passive fever surveillance and seroprevalence studies: protocol of field studies of the Dengue Vaccine Initiative
Abstract
Introduction: Dengue is an important and well-documented public health problem in the Asia-Pacific and Latin American regions. However, in Africa, information on disease burden is limited to case reports and reports of sporadic outbreaks, thus hindering the implementation of public health actions for disease control. To gather evidence on the undocumented burden of dengue in Africa, epidemiological studies with standardised methods were launched in three locations in Africa.
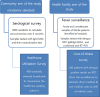
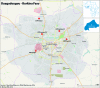
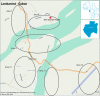
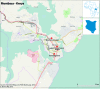
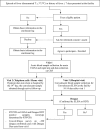
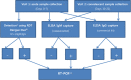
Methods and analysis: In 2014-2017, the Dengue Vaccine Initiative initiated field studies at three sites in Ouagadougou, Burkina Faso; Lambaréné, Gabon and Mombasa, Kenya to obtain comparable incidence data on dengue and assess its burden through standardised hospital-based surveillance and community-based serological methods. Multidisciplinary measurements of the burden of dengue were obtained through field studies that included passive facility-based fever surveillance, cost-of-illness surveys, serological surveys and healthcare utilisation surveys. All three sites conducted case detection using standardised procedures with uniform laboratory assays to diagnose dengue. Healthcare utilisation surveys were conducted to adjust population denominators in incidence calculations for differing healthcare seeking patterns. The fever surveillance data will allow calculation of age-specific incidence rates and comparison of symptomatic presentation between patients with dengue and non-dengue using multivariable logistic regression. Serological surveys assessed changes in immune status of cohorts of approximately 3000 randomly selected residents at each site at 6-month intervals. The age-stratified serosurvey data will allow calculation of seroprevalence and force of infection of dengue. Cost-of-illness evaluations were conducted among patients with acute dengue by Rapid Diagnostic Test.
Ethics and dissemination: By standardising methods to evaluate dengue burden across several sites in Africa, these studies will generate evidence for dengue burden in Africa and data will be disseminated as publication in peer-review journals in 2018.
Keywords: dengue; epidemiology; public health; tropical medicine.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
The STRATAA study protocol: a programme to assess the burden of enteric fever in Bangladesh, Malawi and Nepal using prospective population census, passive surveillance, serological studies and healthcare utilisation surveys.BMJ Open. 2017 Jul 2;7(6):e016283. doi: 10.1136/bmjopen-2017-016283. BMJ Open. 2017. PMID: 28674145 Free PMC article.
-
Epidemiology of dengue fever in Gabon: Results from a health facility-based fever surveillance in Lambaréné and its surroundings.PLoS Negl Trop Dis. 2021 Feb 10;15(2):e0008861. doi: 10.1371/journal.pntd.0008861. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33566822 Free PMC article.
-
The Typhoid Fever Surveillance in Africa Program (TSAP): Clinical, Diagnostic, and Epidemiological Methodologies.Clin Infect Dis. 2016 Mar 15;62 Suppl 1(Suppl 1):S9-S16. doi: 10.1093/cid/civ693. Clin Infect Dis. 2016. PMID: 26933028 Free PMC article.
-
Updated recommendations of the International Dengue Initiative expert group for CYD-TDV vaccine implementation in Latin America.Vaccine. 2019 Oct 8;37(43):6291-6298. doi: 10.1016/j.vaccine.2019.09.010. Epub 2019 Sep 9. Vaccine. 2019. PMID: 31515144 Review.
-
Deconstructing "malaria": West Africa as the next front for dengue fever surveillance and control.Acta Trop. 2014 Jun;134:58-65. doi: 10.1016/j.actatropica.2014.02.017. Epub 2014 Mar 5. Acta Trop. 2014. PMID: 24613157 Review.
Cited by
-
Molecular Evolution of Dengue Virus 3 in Senegal between 2009 and 2022: Dispersal Patterns and Implications for Prevention and Therapeutic Countermeasures.Vaccines (Basel). 2023 Sep 28;11(10):1537. doi: 10.3390/vaccines11101537. Vaccines (Basel). 2023. PMID: 37896941 Free PMC article.
-
First introduction of dengue virus type 3 in Niger, 2022.IJID Reg. 2023 Apr 7;7:230-232. doi: 10.1016/j.ijregi.2023.04.001. eCollection 2023 Jun. IJID Reg. 2023. PMID: 37168517 Free PMC article.
-
Majority of pediatric dengue virus infections in Kenya do not meet 2009 WHO criteria for dengue diagnosis.PLOS Glob Public Health. 2022 Apr 20;2(4):e0000175. doi: 10.1371/journal.pgph.0000175. eCollection 2022. PLOS Glob Public Health. 2022. PMID: 36962138 Free PMC article.
-
Re-Emergence of Dengue Serotype 3 in the Context of a Large Religious Gathering Event in Touba, Senegal.Int J Environ Res Public Health. 2022 Dec 16;19(24):16912. doi: 10.3390/ijerph192416912. Int J Environ Res Public Health. 2022. PMID: 36554793 Free PMC article.
-
Retrospective Genomic Characterization of a 2017 Dengue Virus Outbreak, Burkina Faso.Emerg Infect Dis. 2022 Jun;28(6):1198-1210. doi: 10.3201/eid2806.212491. Emerg Infect Dis. 2022. PMID: 35608626 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

