A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection
- PMID: 29212716
- PMCID: PMC11059970
- DOI: 10.1126/scitranslmed.aan8848
A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection
Abstract
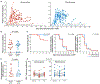
Despite substantial clinical benefits, complete eradication of HIV has not been possible using antiretroviral therapy (ART) alone. Strategies that can either eliminate persistent viral reservoirs or boost host immunity to prevent rebound of virus from these reservoirs after discontinuation of ART are needed; one possibility is therapeutic vaccination. We report the results of a randomized, placebo-controlled trial of a therapeutic vaccine regimen in patients in whom ART was initiated during the early stage of HIV infection and whose immune system was anticipated to be relatively intact. The objectives of our study were to determine whether the vaccine was safe and could induce an immune response that would maintain suppression of plasma viremia after discontinuation of ART. Vaccinations were well tolerated with no serious adverse events but produced only modest augmentation of existing HIV-specific CD4+ T cell responses, with little augmentation of CD8+ T cell responses. Compared with placebo, the vaccination regimen had no significant effect on the kinetics or magnitude of viral rebound after interruption of ART and no impact on the size of the HIV reservoir in the CD4+ T cell compartment. Notably, 26% of subjects in the placebo arm exhibited sustained suppression of viremia (<400 copies/ml) after treatment interruption, a rate of spontaneous suppression higher than previously reported. Our findings regarding the degree and kinetics of plasma viral rebound after ART interruption have potentially important implications for the design of future trials testing interventions aimed at achieving ART-free control of HIV infection.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

Similar articles
-
DNA/MVA Vaccination of HIV-1 Infected Participants with Viral Suppression on Antiretroviral Therapy, followed by Treatment Interruption: Elicitation of Immune Responses without Control of Re-Emergent Virus.PLoS One. 2016 Oct 6;11(10):e0163164. doi: 10.1371/journal.pone.0163164. eCollection 2016. PLoS One. 2016. PMID: 27711228 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection.PLoS One. 2010 May 10;5(5):e10555. doi: 10.1371/journal.pone.0010555. PLoS One. 2010. PMID: 20479938 Free PMC article. Clinical Trial.
-
Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection.J Infect Dis. 2005 Aug 15;192(4):607-17. doi: 10.1086/432002. Epub 2005 Jul 15. J Infect Dis. 2005. PMID: 16028129 Clinical Trial.
-
Harnessing CD8+ T Cells Under HIV Antiretroviral Therapy.Front Immunol. 2019 Feb 26;10:291. doi: 10.3389/fimmu.2019.00291. eCollection 2019. Front Immunol. 2019. PMID: 30863403 Free PMC article. Review.
-
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD006148. doi: 10.1002/14651858.CD006148. Cochrane Database Syst Rev. 2006. PMID: 16856117 Free PMC article. Review.
Cited by
-
Timing of antiretroviral therapy initiation affects intact HIV reservoirs following analytical treatment interruption.J Clin Invest. 2024 Oct 15;134(20):e181632. doi: 10.1172/JCI181632. J Clin Invest. 2024. PMID: 39403920 Free PMC article. No abstract available.
-
Staging of immuno-virological dynamics during acute HIV infection in a Belgian prospective cohort study.J Virus Erad. 2024 Sep 25;10(3):100392. doi: 10.1016/j.jve.2024.100392. eCollection 2024 Sep. J Virus Erad. 2024. PMID: 39403428 Free PMC article.
-
Progress of Astrocyte-Neuron Crosstalk in Central Nervous System Diseases.Neurochem Res. 2024 Dec;49(12):3187-3207. doi: 10.1007/s11064-024-04241-6. Epub 2024 Sep 18. Neurochem Res. 2024. PMID: 39292330 Review.
-
Inhibition of HIV-1 release by ADAM metalloproteinase inhibitors.Front Microbiol. 2024 Mar 20;15:1385775. doi: 10.3389/fmicb.2024.1385775. eCollection 2024. Front Microbiol. 2024. PMID: 38572241 Free PMC article.
-
Impact of antiretroviral therapy during acute or early HIV infection on virologic and immunologic outcomes: results from a multinational clinical trial.AIDS. 2024 Jul 1;38(8):1141-1152. doi: 10.1097/QAD.0000000000003881. Epub 2024 Mar 13. AIDS. 2024. PMID: 38489580 Clinical Trial.
References
-
- Volberding PA, Deeks SG, Antiretroviral therapy and management of HIV infection. Lancet 376, 49–62 (2010). - PubMed
-
- Chun TW, Moir S, Fauci AS, HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol 16, 584–589 (2015). - PubMed
-
- Pantaleo G, Levy Y, Therapeutic vaccines and immunological intervention in HIV infection: a paradigm change. Curr Opin HIV AIDS 11, 576–584 (2016). - PubMed
-
- Pollard RB, Rockstroh JK, Pantaleo G, Asmuth DM, Peters B, Lazzarin A, Garcia F, Ellefsen K, Podzamczer D, van Lunzen J, Arasteh K, Schurmann D, Clotet B, Hardy WD, Mitsuyasu R, Moyle G, Plettenberg A, Fisher M, Fatkenheuer G, Fischl M, Taiwo B, Baksaas I, Jolliffe D, Persson S, Jelmert O, Hovden AO, Sommerfelt MA, Wendel-Hansen V, Sorensen B, Safety and efficacy of the peptide-based therapeutic vaccine for HIV-1, Vacc-4x: a phase 2 randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 14, 291–300 (2014). - PubMed
-
- Goldstein G, Damiano E, Donikyan M, Pasha M, Beckwith E, Chicca J, HIV-1 Tat B-cell epitope vaccination was ineffectual in preventing viral rebound after ART cessation: HIV rebound with current ART appears to be due to infection with new endogenous founder virus and not to resurgence of pre-existing Tat-dependent viremia. Hum Vaccin Immunother 8, 1425–1430 (2012). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials


