How members of the human gut microbiota overcome the sulfation problem posed by glycosaminoglycans
- PMID: 28630303
- PMCID: PMC5502631
- DOI: 10.1073/pnas.1704367114
How members of the human gut microbiota overcome the sulfation problem posed by glycosaminoglycans
Abstract
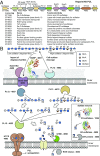
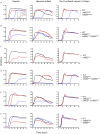
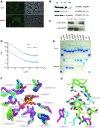
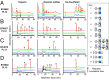
The human microbiota, which plays an important role in health and disease, uses complex carbohydrates as a major source of nutrients. Utilization hierarchy indicates that the host glycosaminoglycans heparin (Hep) and heparan sulfate (HS) are high-priority carbohydrates for Bacteroides thetaiotaomicron, a prominent member of the human microbiota. The sulfation patterns of these glycosaminoglycans are highly variable, which presents a significant enzymatic challenge to the polysaccharide lyases and sulfatases that mediate degradation. It is possible that the bacterium recruits lyases with highly plastic specificities and expresses a repertoire of enzymes that target substructures of the glycosaminoglycans with variable sulfation or that the glycans are desulfated before cleavage by the lyases. To distinguish between these mechanisms, the components of the B. thetaiotaomicron Hep/HS degrading apparatus were analyzed. The data showed that the bacterium expressed a single-surface endo-acting lyase that cleaved HS, reflecting its higher molecular weight compared with Hep. Both Hep and HS oligosaccharides imported into the periplasm were degraded by a repertoire of lyases, with each enzyme displaying specificity for substructures within these glycosaminoglycans that display a different degree of sulfation. Furthermore, the crystal structures of a key surface glycan binding protein, which is able to bind both Hep and HS, and periplasmic sulfatases reveal the major specificity determinants for these proteins. The locus described here is highly conserved within the human gut Bacteroides, indicating that the model developed is of generic relevance to this important microbial community.
Keywords: Bacteroides thetaiotaomicron; glycosaminoglycan degradation; heparan sulfate; heparin; human gut microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Sulfation of Arabinogalactan Proteins Confers Privileged Nutrient Status to Bacteroides plebeius.mBio. 2021 Aug 31;12(4):e0136821. doi: 10.1128/mBio.01368-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340552 Free PMC article.
-
Metabolism of multiple glycosaminoglycans by Bacteroides thetaiotaomicron is orchestrated by a versatile core genetic locus.Nat Commun. 2020 Jan 31;11(1):646. doi: 10.1038/s41467-020-14509-4. Nat Commun. 2020. PMID: 32005816 Free PMC article.
-
Hungatella hathewayi, an Efficient Glycosaminoglycan-Degrading Firmicutes from Human Gut and Its Chondroitin ABC Exolyase with High Activity and Broad Substrate Specificity.Appl Environ Microbiol. 2022 Nov 22;88(22):e0154622. doi: 10.1128/aem.01546-22. Epub 2022 Nov 7. Appl Environ Microbiol. 2022. PMID: 36342199 Free PMC article.
-
Metabolism mechanism of glycosaminoglycans by the gut microbiota: Bacteroides and lactic acid bacteria: A review.Carbohydr Polym. 2024 May 15;332:121905. doi: 10.1016/j.carbpol.2024.121905. Epub 2024 Feb 5. Carbohydr Polym. 2024. PMID: 38431412 Review.
-
Utilization of glycosaminoglycans by the human gut microbiota: participating bacteria and their enzymatic machineries.Gut Microbes. 2022 Jan-Dec;14(1):2068367. doi: 10.1080/19490976.2022.2068367. Gut Microbes. 2022. PMID: 35482895 Free PMC article. Review.
Cited by
-
Candidate genes involved in biosynthesis and degradation of the main extracellular matrix polysaccharides of brown algae and their probable evolutionary history.BMC Genomics. 2024 Oct 10;25(1):950. doi: 10.1186/s12864-024-10811-3. BMC Genomics. 2024. PMID: 39390408 Free PMC article.
-
Limited support for a direct connection between prebiotics and intestinal permeability - a systematic review.Glycoconj J. 2024 Oct;41(4-5):323-342. doi: 10.1007/s10719-024-10165-8. Epub 2024 Sep 17. Glycoconj J. 2024. PMID: 39287885 Free PMC article. Review.
-
The Role of the Gut Microbiota in Sanfilippo Syndrome's Physiopathology: An Approach in Two Affected Siblings.Int J Mol Sci. 2024 Aug 14;25(16):8856. doi: 10.3390/ijms25168856. Int J Mol Sci. 2024. PMID: 39201540 Free PMC article.
-
Barcoded overexpression screens in gut Bacteroidales identify genes with roles in carbon utilization and stress resistance.Nat Commun. 2024 Aug 5;15(1):6618. doi: 10.1038/s41467-024-50124-3. Nat Commun. 2024. PMID: 39103350 Free PMC article.
-
Characterising glycosaminoglycans in human breastmilk and their potential role in infant health.Microb Cell. 2024 Jul 4;11:221-234. doi: 10.15698/mic2024.07.827. eCollection 2024. Microb Cell. 2024. PMID: 38975022 Free PMC article.
References
-
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


