Enhanced Tau Aggregation in the Presence of Amyloid β
- PMID: 28500862
- PMCID: PMC5500829
- DOI: 10.1016/j.ajpath.2017.03.011
Enhanced Tau Aggregation in the Presence of Amyloid β
Abstract
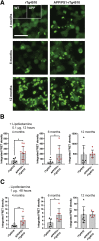
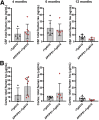
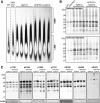
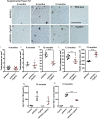
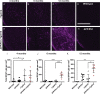
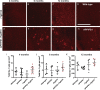
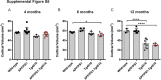
Amyloid plaques and neurofibrillary tangles co-occur in Alzheimer disease, but with different topological and temporal patterns. Whether these two lesions are independent or pathobiologically related is uncertain. For example, amyloid deposition in the neocortex precedes the spread of tau neurofibrillary tangles from the limbic areas to the cortex. We examined the aggregation properties of tau isolated from human cases with early tau pathology (Braak II) with and without plaques. Using a well-established HEK cell biosensor assay, we show that tau from cases with plaques has an enhanced ability to induce tau aggregates compared to tau from cases without plaques. To further explore this effect, we combined mice carrying the APP/PS1 transgene array that develop plaques with rTg4510 mice carrying the P301L mutant human tau transgene that develop extensive tau pathology with age. The resulting APP/PS1-rTg4510 mice had a threefold increase in tau seeding activity over the rTg4510 strain, without change in tau production or extracellular release. Surprisingly, this effect was observed before overt amyloid deposition. The enhancement of tau aggregation was also apparent by an increase in histological measures of tau pathology in young APP/PS1-rTg4510 mice and an increase in high-molecular-weight tau. Overall, these data provide evidence that amyloid β acts to enhance tau pathology by increasing the formation of tau species capable of seeding new aggregates.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Tau reduction in the presence of amyloid-β prevents tau pathology and neuronal death in vivo.Brain. 2018 Jul 1;141(7):2194-2212. doi: 10.1093/brain/awy117. Brain. 2018. PMID: 29733334 Free PMC article.
-
Human tau increases amyloid β plaque size but not amyloid β-mediated synapse loss in a novel mouse model of Alzheimer's disease.Eur J Neurosci. 2016 Dec;44(12):3056-3066. doi: 10.1111/ejn.13442. Epub 2016 Nov 12. Eur J Neurosci. 2016. PMID: 27748574 Free PMC article.
-
Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer's disease mice.J Neurosci. 2010 Jul 7;30(27):9228-40. doi: 10.1523/JNEUROSCI.0418-10.2010. J Neurosci. 2010. PMID: 20610758 Free PMC article.
-
Review on Alzheimer's disease: Inhibition of amyloid beta and tau tangle formation.Int J Biol Macromol. 2021 Jan 15;167:382-394. doi: 10.1016/j.ijbiomac.2020.11.192. Epub 2020 Dec 2. Int J Biol Macromol. 2021. PMID: 33278431 Review.
-
Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models.Int J Dev Neurosci. 2004 Nov;22(7):453-65. doi: 10.1016/j.ijdevneu.2004.07.013. Int J Dev Neurosci. 2004. PMID: 15465275 Review.
Cited by
-
MAD-microbial (origin of) Alzheimer's disease hypothesis: from infection and the antimicrobial response to disruption of key copper-based systems.Front Neurosci. 2024 Oct 2;18:1467333. doi: 10.3389/fnins.2024.1467333. eCollection 2024. Front Neurosci. 2024. PMID: 39416952 Free PMC article. Review.
-
Sequence of Molecular Events in the Development of Alzheimer's Disease: Cascade Interactions from Beta-Amyloid to Other Involved Proteins.Cells. 2024 Jul 31;13(15):1293. doi: 10.3390/cells13151293. Cells. 2024. PMID: 39120323 Free PMC article. Review.
-
Nanoligomers targeting NF-κB and NLRP3 reduce neuroinflammation and improve cognitive function with aging and tauopathy.J Neuroinflammation. 2024 Jul 27;21(1):182. doi: 10.1186/s12974-024-03182-9. J Neuroinflammation. 2024. PMID: 39068433 Free PMC article.
-
Spatial extent as a sensitive amyloid-PET metric in preclinical Alzheimer's disease.Alzheimers Dement. 2024 Aug;20(8):5434-5449. doi: 10.1002/alz.14036. Epub 2024 Jul 10. Alzheimers Dement. 2024. PMID: 38988055 Free PMC article.
-
FGFR3 drives Aβ-induced tau uptake.Exp Mol Med. 2024 Jul;56(7):1631-1642. doi: 10.1038/s12276-024-01274-3. Epub 2024 Jul 1. Exp Mol Med. 2024. PMID: 38951140 Free PMC article.
References
-
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Thal D.R., Rüb U., Orantes M., Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. - PubMed
-
- Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. - PubMed
-
- Bierer L.M., Hof P.R., Purohit D.P., Carlin L., Schmeidler J., Davis K.L., Perl D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol. 1995;52:81–88. - PubMed
-
- Bolmont T., Clavaguera F., Meyer-Luehmann M., Herzig M.C., Radde R., Staufenbiel M., Lewis J., Hutton M., Tolnay M., Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP × Tau transgenic mice. Am J Pathol. 2007;171:2012–2020. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous


