The desert gerbil Psammomys obesus as a model for metformin-sensitive nutritional type 2 diabetes to protect hepatocellular metabolic damage: Impact of mitochondrial redox state
- PMID: 28222147
- PMCID: PMC5319739
- DOI: 10.1371/journal.pone.0172053
The desert gerbil Psammomys obesus as a model for metformin-sensitive nutritional type 2 diabetes to protect hepatocellular metabolic damage: Impact of mitochondrial redox state
Abstract
Introduction: While metformin (MET) is the most widely prescribed antidiabetic drug worldwide, its beneficial effects in Psammomys obesus (P. obesus), a rodent model that mimics most of the metabolic features of human diabetes, have not been explored thoroughly. Here, we sought to investigate whether MET might improve insulin sensitivity, glucose homeostasis, lipid profile as well as cellular redox and energy balance in P. obesus maintained on a high energy diet (HED).
Materials and methods: P. obesus gerbils were randomly assigned to receive either a natural diet (ND) consisting of halophytic plants (control group) or a HED (diabetic group) for a period of 24 weeks. MET (50 mg/kg per os) was administered in both animal groups after 12 weeks of feeding, i.e., the time required for the manifestation of insulin resistance in P. obesus fed a HED. Parallel in vitro experiments were conducted on isolated hepatocytes that were shortly incubated (30 min) with MET and energetic substrates (lactate + pyruvate or alanine, in the presence of octanoate).
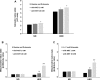
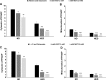
Results: In vivo, MET lowered glycemia, glycosylated haemoglobin, circulating insulin and fatty acid levels in diabetic P. obesus. It also largely reversed HED-induced hepatic lipid alterations. In vitro, MET increased glycolysis but decreased both gluconeogenesis and ketogenesis in the presence of glucogenic precursors and medium-chain fatty acid. Importantly, these changes were associated with an increase in cytosolic and mitochondrial redox states along with a decline in respiration capacity.
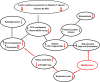
Conclusions: MET prevents the progression of insulin resistance in diabetes-prone P. obesus, possibly through a tight control of gluconeogenesis and fatty acid β-oxidation depending upon mitochondrial function. While the latter is increasingly becoming a therapeutic issue in diabetes, the gut microbiota is another promising target that would need to be considered as well.
Conflict of interest statement
Figures
Similar articles
-
Beneficial effects of silibinin against the progression of metabolic syndrome, increased oxidative stress, and liver steatosis in Psammomys obesus, a relevant animal model of human obesity and diabetes.J Diabetes. 2014 Mar;6(2):184-92. doi: 10.1111/1753-0407.12083. Epub 2013 Sep 30. J Diabetes. 2014. PMID: 23953934
-
Potential Applications of Thyroid Hormone Derivatives in Obesity and Type 2 Diabetes: Focus on 3,5-Diiodothyronine (3,5-T2) in Psammomys obesus (Fat Sand Rat) Model.Nutrients. 2022 Jul 25;14(15):3044. doi: 10.3390/nu14153044. Nutrients. 2022. PMID: 35893898 Free PMC article.
-
Nutritionally induced diabetes in desert rodents as models of type 2 diabetes: Acomys cahirinus (spiny mice) and Psammomys obesus (desert gerbil).ILAR J. 2006;47(3):212-24. doi: 10.1093/ilar.47.3.212. ILAR J. 2006. PMID: 16804196 Review.
-
Treatment of diabetes with vanadium salts: general overview and amelioration of nutritionally induced diabetes in the Psammomys obesus gerbil.Diabetes Metab Res Rev. 2001 Jan-Feb;17(1):55-66. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr165>3.0.co;2-j. Diabetes Metab Res Rev. 2001. PMID: 11241892 Review.
-
Regulation of muscle malonyl-CoA levels in the nutritionally insulin-resistant desert gerbil, Psammomys obesus.Diabetes Metab Res Rev. 2002 May-Jun;18(3):217-23. doi: 10.1002/dmrr.288. Diabetes Metab Res Rev. 2002. PMID: 12112940
Cited by
-
Progress in experimental models to investigate the in vivo and in vitro antidiabetic activity of drugs.Animal Model Exp Med. 2024 Jun;7(3):297-309. doi: 10.1002/ame2.12442. Epub 2024 Jun 4. Animal Model Exp Med. 2024. PMID: 38837635 Free PMC article. Review.
-
Role of glycolysis in diabetic atherosclerosis.World J Diabetes. 2023 Oct 15;14(10):1478-1492. doi: 10.4239/wjd.v14.i10.1478. World J Diabetes. 2023. PMID: 37970130 Free PMC article. Review.
-
Anti-Obesity and Anti-Dyslipidemic Effects of Salicornia arabica Decocted Extract in Tunisian Psammomys obesus Fed a High-Calorie Diet.Foods. 2023 Mar 11;12(6):1185. doi: 10.3390/foods12061185. Foods. 2023. PMID: 36981112 Free PMC article.
-
Species Differences in Tryptophan Metabolism and Disposition.Int J Tryptophan Res. 2022 Oct 29;15:11786469221122511. doi: 10.1177/11786469221122511. eCollection 2022. Int J Tryptophan Res. 2022. PMID: 36325027 Free PMC article. Review.
-
Pup ultrasonic isolation calls of six gerbil species and the relationship between acoustic traits and body size.R Soc Open Sci. 2021 Mar 3;8(3):201558. doi: 10.1098/rsos.201558. R Soc Open Sci. 2021. PMID: 33959325 Free PMC article.
References
-
- King H, Aubert RE, Herman WH. Global Burden of Diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21(9): 1414–1431. - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55(6): 1577–1596. 10.1007/s00125-012-2534-0 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


