Vitamin D for the management of asthma
- PMID: 27595415
- PMCID: PMC6457769
- DOI: 10.1002/14651858.CD011511.pub2
Vitamin D for the management of asthma
Update in
-
Vitamin D for the management of asthma.Cochrane Database Syst Rev. 2023 Feb 6;2(2):CD011511. doi: 10.1002/14651858.CD011511.pub3. Cochrane Database Syst Rev. 2023. PMID: 36744416 Free PMC article. Review.
Abstract
Background: Several clinical trials of vitamin D to prevent asthma exacerbation and improve asthma control have been conducted in children and adults, but a meta-analysis restricted to double-blind, randomised, placebo-controlled trials of this intervention is lacking.
Objectives: To evaluate the efficacy of administration of vitamin D and its hydroxylated metabolites in reducing the risk of severe asthma exacerbations (defined as those requiring treatment with systemic corticosteroids) and improving asthma symptom control.
Search methods: We searched the Cochrane Airways Group Trial Register and reference lists of articles. We contacted the authors of studies in order to identify additional trials. Date of last search: January 2016.
Selection criteria: Double-blind, randomised, placebo-controlled trials of vitamin D in children and adults with asthma evaluating exacerbation risk or asthma symptom control or both.
Data collection and analysis: Two review authors independently applied study inclusion criteria, extracted the data, and assessed risk of bias. We obtained missing data from the authors where possible. We reported results with 95% confidence intervals (CIs).
Main results: We included seven trials involving a total of 435 children and two trials involving a total of 658 adults in the primary analysis. Of these, one trial involving 22 children and two trials involving 658 adults contributed to the analysis of the rate of exacerbations requiring systemic corticosteroids. Duration of trials ranged from four to 12 months, and the majority of participants had mild to moderate asthma. Administration of vitamin D reduced the rate of exacerbations requiring systemic corticosteroids (rate ratio 0.63, 95% CI 0.45 to 0.88; 680 participants; 3 studies; high-quality evidence), and decreased the risk of having at least one exacerbation requiring an emergency department visit or hospitalisation or both (odds ratio (OR) 0.39, 95% CI 0.19 to 0.78; number needed to treat for an additional beneficial outcome, 27; 963 participants; 7 studies; high-quality evidence). There was no effect of vitamin D on % predicted forced expiratory volume in one second (mean difference (MD) 0.48, 95% CI -0.93 to 1.89; 387 participants; 4 studies; high-quality evidence) or Asthma Control Test scores (MD -0.08, 95% CI -0.70 to 0.54; 713 participants; 3 studies; high-quality evidence). Administration of vitamin D did not influence the risk of serious adverse events (OR 1.01, 95% CI 0.54 to 1.89; 879 participants; 5 studies; moderate-quality evidence). One trial comparing low-dose versus high-dose vitamin D reported two episodes of hypercalciuria, one in each study arm. No other study reported any adverse event potentially attributable to administration of vitamin D. No participant in any included trial suffered a fatal asthma exacerbation. We did not perform a subgroup analysis to determine whether the effect of vitamin D on risk of severe exacerbation was modified by baseline vitamin D status, due to unavailability of suitably disaggregated data. We assessed two trials as being at high risk of bias in at least one domain; neither trial contributed data to the analysis of the outcomes reported above.
Authors' conclusions: Meta-analysis of a modest number of trials in people with predominantly mild to moderate asthma suggests that vitamin D is likely to reduce both the risk of severe asthma exacerbation and healthcare use. It is as yet unclear whether these effects are confined to people with lower baseline vitamin D status; further research, including individual patient data meta-analysis of existing datasets, is needed to clarify this issue. Children and people with frequent severe asthma exacerbations were under-represented; additional primary trials are needed to establish whether vitamin D can reduce the risk of severe asthma exacerbation in these groups.
Conflict of interest statement
ARM, MU, MJ, and CJG all acted as investigators in one or more clinical trials contributing data to this review. The 'Risk of bias' assessment for the study authored by ARM and CJG was performed independently by UN and CJC (Martineau 2015). For all other studies, ARM and one of CJC and APG independently assessed the risk of bias for each study. Where data from primary studies conducted by review authors contributed to a given outcome, the quality of the evidence was assessed by review authors who were not involved with those primary studies (CJC and AS).
Figures
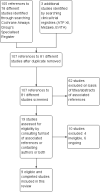

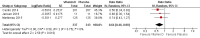
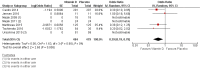

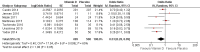




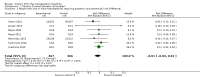


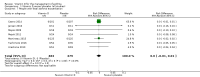


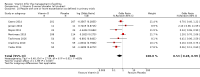





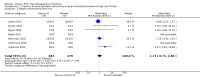
Comment in
-
Review: In adults with asthma, vitamin D3 reduces rate of severe exacerbation but does not improve symptoms.Ann Intern Med. 2016 Dec 20;165(12):JC66. doi: 10.7326/ACPJC-2016-165-12-066. Ann Intern Med. 2016. PMID: 27992922 No abstract available.
Similar articles
-
Vitamin D for the management of asthma.Cochrane Database Syst Rev. 2023 Feb 6;2(2):CD011511. doi: 10.1002/14651858.CD011511.pub3. Cochrane Database Syst Rev. 2023. PMID: 36744416 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Vitamin D for the management of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2024 Sep 27;9(9):CD013284. doi: 10.1002/14651858.CD013284.pub2. Cochrane Database Syst Rev. 2024. PMID: 39329240
-
Antibiotics for exacerbations of asthma.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD002741. doi: 10.1002/14651858.CD002741.pub2. Cochrane Database Syst Rev. 2018. PMID: 29938789 Free PMC article. Review.
-
Macrolides versus placebo for chronic asthma.Cochrane Database Syst Rev. 2021 Nov 22;11(11):CD002997. doi: 10.1002/14651858.CD002997.pub5. Cochrane Database Syst Rev. 2021. PMID: 34807989 Free PMC article. Review.
Cited by
-
A 12-week double-blind randomised controlled trial investigating the effect of dietary supplementation with 125 μg/d vitamin D in adults with asthma.Br J Nutr. 2024 Sep 28;132(6):738-749. doi: 10.1017/S0007114524000953. Epub 2024 May 16. Br J Nutr. 2024. PMID: 38751303 Free PMC article. Clinical Trial.
-
The effects of vitamin D supplementation on inflammatory biomarkers in patients with asthma: a systematic review and meta-analysis of randomized controlled trials.Front Immunol. 2024 Mar 13;15:1335968. doi: 10.3389/fimmu.2024.1335968. eCollection 2024. Front Immunol. 2024. PMID: 38545098 Free PMC article.
-
Association of serum vitamin D concentration with the final course of hospitalization in patients with COVID-19.Front Immunol. 2023 Sep 1;14:1231813. doi: 10.3389/fimmu.2023.1231813. eCollection 2023. Front Immunol. 2023. PMID: 37727794 Free PMC article.
-
Maintenance Therapy for Children and Adolescents with Asthma: Guidelines and Recommendations from the Emilia-Romagna Asthma (ERA) Study Group.J Clin Med. 2023 Aug 23;12(17):5467. doi: 10.3390/jcm12175467. J Clin Med. 2023. PMID: 37685533 Free PMC article.
-
Vitamin D Levels in the Pre- and Post-COVID-19 Pandemic Periods and Related Confinement at Pediatric Age.Nutrients. 2023 Apr 26;15(9):2089. doi: 10.3390/nu15092089. Nutrients. 2023. PMID: 37432239 Free PMC article.
References
References to studies included in this review
Castro 2014 {published data only}
-
- Jiao J, King TS, McKenzie M, Bacharier LB, Dixon AE, Codispoti CD, et al. Effects of vitamin D3 supplementation in adults with asthma complicated by sinonasal disease. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A4148.
-
- Jiao J, King TS, McKenzie M, Bacharier LB, Dixon AE, Codispoti CD, et al. Vitamin D3 therapy in patients with asthma complicated by sinonasal disease: Secondary analysis of the Vitamin D Add‐on Therapy Enhances Corticosteroid Responsiveness in Asthma trial. Journal of Allergy and Clinical Immunology 2016;138(2):589‐92. - PMC - PubMed
Jensen 2016 {unpublished data only}
Lewis 2012 {published data only}
-
- Lewis E, Fernandez C, Nella A, Hopp R, Gallagher JC, Casale TB. Relationship of 25‐hydroxyvitamin D and asthma control in children. Annals of Allergy, Asthma & Immunology 2012;108(4):281‐2. - PubMed
-
- Lewis E, Fernandez C, Nella AA, Hopp R, Casale T, Gallagher C. The relationship of vitamin D and asthma in children. Journal of Allergy and Clinical Immunology. 2011; Vol. 127:Suppl 1.
Majak 2009 {published data only}
-
- Majak P, Jerzyiska J, Smejda K, Stelmach I, Timler D, Stelmach W. Correlation of vitamin D with Foxp3 induction and steroid‐sparing effect of immunotherapy in asthmatic children. Annals of Allergy, Asthma & Immunology 2012;109(5):329‐35. - PubMed
-
- Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clinical & Experimental Allergy 2009;39(12):1830‐41. - PubMed
Majak 2011 {published data only}
-
- Majak P, Olszowiec‐Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. Journal of Allergy and Clinical Immunology 2011;127(5):1294‐6. - PubMed
Martineau 2015 {published data only}
-
- Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Choudhury AB, et al. Double‐blind multi‐centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid‐treated asthma (ViDiAs). Thorax. 2014:A51‐2.
-
- Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Double‐blind randomised placebo‐controlled trial of bolus‐dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015;70(5):451‐7. - PubMed
Tachimoto 2016 {published and unpublished data}
-
- Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved control of childhood asthma with low‐dose, short‐term vitamin D supplementation: a randomized, double‐blind, placebo‐controlled trial. Allergy 2016;71(7):1001‐9. - PubMed
Urashima 2010 {published data only}
-
- Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. The American Journal of Clinical Nutrition 2010;91(5):1255‐60. - PubMed
Yadav 2014 {published data only}
-
- Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian Journal of Pediatrics 2014;81(7):650‐4. - PubMed
References to studies excluded from this review
Alansari 2015 {published data only}
-
- Alansari K, Alattar M, Ibrahim KY, Davidson BL, Elnair MB, Mohamed AN. A randomized trial of vitamin D to reduce pediatric asthma exacerbations. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A2621.
Arshi 2014 {published data only}
-
- Arshi S, Fallahpour M, Nabavi M, Bemanian MH, Javad‐Mousavi SA, Nojomi M, et al. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthma. Annals of Allergy, Asthma & Immunology 2014;113(4):404‐9. - PubMed
Bantz 2015 {published data only}
-
- Bantz SK, Dy T, Herzog R. Vitamin D deficiency in a young, atopic pediatric population. Journal of Allergy and Clinical Immunology. 2015; Vol. 135 (2 Suppl 1):AB148.
Baris 2014 {published data only}
-
- Baris S, Kiykim A, Ozen A, Tulunay A, Karakoc‐Aydiner E, Barlan IB. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy 2014;69(2):246‐53. - PubMed
-
- Safa B, Karakoc‐Aydiner E, Cagan H, Kiykim A, Tulunay A, Akkoc T, et al. 25 (OH) vitamin D3 as an adjunct to subcutaneous allergen immunotherapy: Is it effective?. 31st Congress of the European Academy of Allergy and Clinical Immunology; 2012 June 16‐20; Geneva. 2012.
Bar Yoseph 2015 {published data only}
-
- Bar‐Yoseph R, Livnat G, Schnapp Z, Dabbah H, Goldbart A, Bentur LPY. The effect of vitamin D therapy on airway reactivity and airway inflammation in asthmatic children. European Respiratory Society Annual Congress; 2013 Sept 7‐11; Barcelona, Spain. 2013; Vol. 42, Suppl 57:1159.
Breitenbuecher 2012 {published data only}
-
- Breitenbuecher A, Voit U, Miedinger D, Chhajed P, Krapf R, Leuppi J. Calcitriol‐treatment in patients with severe persistent asthma: A randomized, placebo‐controlled study. Respiration; International Review of Thoracic Diseases 2014;87(6):523‐4.
-
- Breitenbuecher A, Voit U, Miedinger D, Leuppi J, Krapf R. Substitution of vitamin D in patients with moderate to severe persistent asthma: A randomized, placebo‐controlled pilot study. European Respiratory Journal 2012;40:Suppl 56 4698.
Darabi 2013 {published data only}
-
- Darabi B, Moin M, Pourpak Z. The effect of vitamin D supplementation over asthma outcome. 2nd International Congress of Immunology; 2013 Feb 19‐21; Tehran. 2013.
De Groot 2015 {published data only}
-
- Groot JC, Roon ENH, Storm H, Veeger NJ, Bel EHD, Brinke A. The effect of vitamin D on airway inflammation in non‐atopic asthma. American Journal of Respiratory and Critical Care Medicine. 2014; Vol. 189:A1390.
-
- Groot JC, Roon EN, Storm H, Veeger NJ, Zwinderman AH, Hiemstra PS, et al. Vitamin D reduces eosinophilic airway inflammation in non‐atopic asthma. Journal of Allergy and Clinical Immunology 2015;135(3):670‐5. - PubMed
-
- Groot JC, Roon EN, Storm H, Bel EH, Brinke A. The effect of a single high dose vitamin D3 on neutrophilic airway inflammation in nonatopic asthma. European Respiratory Journal. 2012; Vol. 40 Suppl 56:P1790.
Goldring 2013 {published data only}
Lakatos 2000 {published data only}
-
- Lakatos P, Nagy Z, Kiss L, Horvath C, Takacs I, Foldes J, et al. Prevention of corticosteroid‐induced osteoporosis by alfacalcidol. Zeitschrift fur Rheumatologie. 2000; Vol. 59 (Suppl 1):48‐52. - PubMed
Litonjua 2014 {published data only}
-
- Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemporary Clinical Trials 2014;38(1):37‐50. - PMC - PubMed
McDonald 2006 {published data only}
-
- McDonald CF, Matthews S, Seeman E. A two year double blind placebo controlled prospective study of the effects of calcitriol on bone mineral density (BMD) in patients with asthma. Proceedings of the Thoracic Society of Australia & New Zealand, Annual Scientific Meeting; 2003 April 4‐9; Adelaide. 2003:P022.
-
- McDonald CF, Zebaze RMD, Seeman E. Calcitriol does not prevent bone loss in patients with asthma receiving corticosteroid therapy: a double‐blind placebo‐controlled trial. Osteoporosis International 2006;17(10):1546‐51. - PubMed
Menon 2014 {published data only}
-
- Menon B, Nima G, Dogra V, Mittal A, Kaur C, Mittal U. Evaluation of vitamin D in bronchial asthma and the effect of vitamin D supplementation on asthma severity and control: A randomised control trial. European Respiratory Journal. 2014; Vol. 44 Suppl 58:P4049.
Nanzer 2014 {published data only}
-
- Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Martineau AR, Griffiths CJ, et al. Calcitriol restores glucocorticoid responsiveness in steroid resistant asthmatics through reduction of IL‐17A. Immunology. 2014; Vol. 143:50.
-
- Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, et al. Distinct endotypes of steroid‐resistant asthma characterized by IL‐17A (high) and IFN‐gamma (high) immunophenotypes: Potential benefits of calcitriol. Journal of Allergy and Clinical Immunology 2015;136(3):628‐37. - PMC - PubMed
-
- Chambers ES, Nanzer AM, Richards DF, Freeman A, Ryanna K, Griffiths C, et al. Serum 25(OH)D levels can predict Foxp3+ treg frequency and steroid responsiveness in severe asthmatics. Immunology 2011;135:S1.
-
- Nanzer AM, Chambers ES, Ryanna K, Freeman AT, Colligan G, Richards DF, et al. The effects of calcitriol treatment in glucocorticoid‐resistant asthma. Journal of Allergy and Clinical Immunology 2014;133(6):1755‐7. - PubMed
Price 2015 {published data only}
-
- Price OJ, Hull JH, Howatson G, Robson‐Ansley P, Ansley L. Vitamin D and omega‐3 polyunsaturated fatty acid supplementation in athletes with exercise‐induced bronchoconstriction: A pilot study. Expert Review of Respiratory Medicine 2015;9(3):369‐78. - PubMed
Rajanandh 2015 {published data only}
Schou 2003 {published data only}
-
- Schou AJ, Heuck C, Wolthers OD. Does vitamin D administered to children with asthma treated with inhaled glucocorticoids affect short‐term growth or bone turnover?. Pediatric Pulmonology 2003;36(5):399‐404. - PubMed
-
- Wolthers OD, Schou AJ, Heuck C. A double blind trial of vitamin‐D in children with asthma treated with inhaled budesonide. European Respiratory Society 9th Annual Congress; 1999 Oct 9‐13; Madrid. 1999.
Thijs 2011 {published data only}
-
- Thijs W, Janssen K, Verhoosel RM, Papapoulos SE, Cessie S, Middeldorp S, et al. Effect of vitamin D treatment on antimicrobial peptides in asthma patients and healthy controls. ERJ 2011;38(55):4888.
Torres 2013 {published data only}
-
- Torres J, Martinez M, Chavez E, Garcia D, Fabiano F, Hernandez J. Vitamin D levels in peripheral blood in patients with mild to moderate bronchial asthma. European Academy of Allergy and Clinical Immunology and World Allergy Organization World Allergy and Asthma Congress; 2013 June 22‐26; Milan Italy. 2013:68.
Utz 1976 {published data only}
-
- Utz G, Hauck AM. Oral application of calcium and vitamin D2 in allergic bronchial asthma. MMW Munch Med Wochenschr 1976;118(43):1395‐8. - PubMed
Worth 1994 {published data only}
-
- Worth H, Stammen D, Keck E. Therapy of steroid‐induced bone loss in adult asthmatics with calcium, vitamin D, and a diphosphonate. American Journal of Respiratory and Critical Care Medicine 1994;150(2):394‐7. - PubMed
Yemelyanov 2001 {published data only}
-
- Yemelyanov A, Shevelev S, Murzin B, Shubin S. Efficacy and safety of calcium and vitamin D in treatment of steroid osteoporosis in asthmatic patients. European Respiratory Journal 2001;18:S33.
References to ongoing studies
NCT01419262 {published data only}
-
- NCT01419262. DO IT Trial: Vitamin D outcomes and interventions in toddlers (DO IT). https://clinicaltrials.gov/ct2/show/NCT01419262 (accessed 11 August 2016). - PubMed
NCT01728571 {published data only}
-
- NCT01728571. LungVITamin D and OmegA‐3 Trial (lungVITAL) (lungVITAL). https://clinicaltrials.gov/ct2/show/NCT01728571 (accessed 11 August 2016).
NCT02197702 {published data only}
-
- NCT02197702. Vitamin D in preschoolers with viral‐induced asthma (DIVA). https://clinicaltrials.gov/ct2/show/NCT02197702 (accessed 11 August 2016).
NCT02424552 {published data only}
-
- NCT02424552. Effect of vitamin D as add‐on therapy for vitamin D insufficient patients with severe asthma (EVITA). https://clinicaltrials.gov/ct2/show/NCT02424552 (accessed 11 August 2016).
NCT02428322 {published data only}
-
- Hutchinson K, Kerley C, Elnazir B, Couglan D, Greally P, Rochev Y, et al. A randomized, double‐blind, placebo‐controlled trial of vitamin D for Irish children with asthma: Baseline data. Irish Journal of Medical Science. 2014; Vol. 183 (9 Suppl 1):S443.
-
- Kerley C, Greally P, Coughlan D, Elnazir BPY. A randomized, double‐blind, placebo‐controlled trial of vitamin D3 for Irish children with asthma: Baseline data. European Respiratory Journal. 2014; Vol. 44, Suppl 58:1172.
-
- Kerley CP, Hutchinson K, Greally P, Coghlan D, Elnazir B. The effects of vitamin D supplementation on pulmonary function, disease severity and markers of inflammation in childhood asthmatics: A randomized, double‐blind, placebo‐controlled trial. Irish Journal of Medical Science. 2014; Vol. 183 11 Suppl 1:S522.
-
- NCT02428322. Trial of Vitamin D3 Supplementation in Paediatric Asthma (NCHVitDAst). https://clinicaltrials.gov/ct2/show/NCT02428322 (accessed 11 August 2016).
Patella 2013 {published data only}
-
- Florio G, Rubano A, Patella V. Effect of sublingual specific immunotherapy associated to vitamin D3 and lactobacillus reuteri in asthmatic teens. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A4269.
-
- Patella V, Florio G, Palmieri M. Vitamin D3 associated to lactobacillus reuteri improves effects of allergen immunotherapy in asthmatic children. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013.
UMIN000004160 {published data only}
-
- UMIN000004160. A randomized, double blind, comparative study of vitamin D3 versus placebo in small children with asthma to prevent asthma attack. https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_his_list.cgi?recptno=R0... (accessed 11 August 2016).
Additional references
AVID‐Asthma IPDMA
-
- Martineau AR, Jolliffe DA, Hooper RL, Khan KS, Griffiths CJ, Camargo CA Jr. Individual patient data meta‐analysis of randomised controlled trials of vitamin D supplementation to prevent acute respiratory infection and acute exacerbations of asthma and COPD. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013953 (accessed 11 August 2016).
Brehm 2010
Brehm 2012
Confino‐Cohen 2014
-
- Confino‐Cohen R, Brufman I, Goldberg A, Feldman BS. Vitamin D, asthma prevalence and asthma exacerbations: a large adult population‐based study. Allergy 2014;69(12):1673‐80. - PubMed
Coussens 2012
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 11 August 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Greiller 2015
Heaney 2003
-
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger‐Lux MJ. Human serum 25‐hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition 2003;77:204‐10. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at www.cochrane‐handbook.org.
Holick 2007
-
- Holick MF. Vitamin D deficiency. The New England Journal of Medicine 2007;357(3):266‐81. - PubMed
Hollis 2013
Janssens 2013
-
- Janssens W, Decramer M, Mathieu C, Korf H. Vitamin D and chronic obstructive pulmonary disease: hype or reality?. The Lancet Respiratory Medicine 2013;1(10):804‐12. - PubMed
Johnston 2006
Jones 2005
-
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75‐9. - PubMed
Lai 2009
-
- Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476‐83. - PubMed
Lan 2014
Lehouck 2012
-
- Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Eldere J, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease. Annals of Internal Medicine 2012;156:105‐14. - PubMed
Luo 2015
Mann 2014
-
- Mann EH, Chambers ES, Pfeffer PE, Hawrylowicz CM. Immunoregulatory mechanisms of vitamin D relevant to respiratory health and asthma. Annals of the New York Academy of Sciences 2014;1317:57‐69. - PubMed
Martineau 2007
-
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, et al. IFN‐gamma‐ and TNF‐independent vitamin D‐inducible human suppression of mycobacteria: the role of cathelicidin LL‐37. Journal of Immunology 2007;178(11):7190‐8. - PubMed
Martineau 2012
-
- Martineau AR. Bolus‐dose vitamin D and prevention of childhood pneumonia. The Lancet 2012;379(9824):1373‐5. - PubMed
Martineau 2015a
-
- Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double‐blind, randomised controlled trial. The Lancet Respiratory Medicine 2015;3(2):120‐30. - PubMed
Martineau 2015b
Moher 2009
Reddel 2009
-
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. American Journal of Respiratory and Critical Care Medicine 2009;180(1):59‐99. - PubMed
RevMan 2015 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Riverin 2015
Romagnoli 2008
-
- Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, et al. Short and long‐term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. Journal of Clinical Endocrinology and Metabolism 2008;93(8):3015‐20. - PubMed
Singh 2006
To 2012
Vieth 2009
-
- Vieth R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Research 2009;29(9):3675‐84. - PubMed
Xiao 2015
-
- Xiao L, Xing C, Yang Z, Xu S, Wang M, Du H, et al. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. British Journal of Nutrition 2015;114:1026‐34. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


