Evolutionary patterns in coiled-coils
- PMID: 25577198
- PMCID: PMC4350178
- DOI: 10.1093/gbe/evv007
Evolutionary patterns in coiled-coils
Abstract
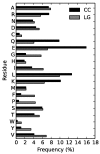
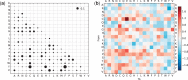
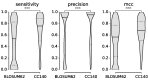
Models of protein evolution are used to describe evolutionary processes, for phylogenetic analyses and homology detection. Widely used general models of protein evolution are biased toward globular domains and lack resolution to describe evolutionary processes for other protein types. As three-dimensional structure is a major constraint to protein evolution, specific models have been proposed for other types of proteins. Here, we consider evolutionary patterns in coiled-coil forming proteins. Coiled-coils are widespread structural domains, formed by a repeated motif of seven amino acids (heptad repeat). Coiled-coil forming proteins are frequently rods and spacers, structuring both the intracellular and the extracellular spaces that often form protein interaction interfaces. We tested the hypothesis that due to their specific structure the associated evolutionary constraints differ from those of globular proteins. We showed that substitution patterns in coiled-coil regions are different than those observed in globular regions, beyond the simple heptad repeat. Based on these substitution patterns we developed a coiled-coil specific (CC) model that in the context of phylogenetic reconstruction outperforms general models in tree likelihood, often leading to different topologies. For multidomain proteins containing both a coiled-coil region and a globular domain, we showed that a combination of the CC model and a general one gives higher likelihoods than a single model. Finally, we showed that the model can be used for homology detection to increase search sensitivity for coiled-coil proteins. The CC model, software, and other supplementary materials are available at http://www.evocell.org/cgl/resources (last accessed January 29, 2015).
Keywords: amino acid substitutions; coiled-coil; homology detection; phylogenetic inference; protein evolution; protein structure.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures



Similar articles
-
Socket: a program for identifying and analysing coiled-coil motifs within protein structures.J Mol Biol. 2001 Apr 13;307(5):1427-50. doi: 10.1006/jmbi.2001.4545. J Mol Biol. 2001. PMID: 11292353
-
Effects of side-chain characteristics on stability and oligomerization state of a de novo-designed model coiled-coil: 20 amino acid substitutions in position "d".J Mol Biol. 2000 Jul 7;300(2):377-402. doi: 10.1006/jmbi.2000.3866. J Mol Biol. 2000. PMID: 10873472
-
Model structure of the Omp alpha rod, a parallel four-stranded coiled coil from the hyperthermophilic eubacterium Thermotoga maritima.J Mol Biol. 1995 Apr 21;248(1):180-9. doi: 10.1006/jmbi.1995.0210. J Mol Biol. 1995. PMID: 7731042
-
Phylogenetic analysis of membrane trafficking proteins: a family reunion and secondary structure predictions.Eur J Cell Biol. 1997 Jul;73(3):198-204. Eur J Cell Biol. 1997. PMID: 9243180 Review.
-
Pharmacological interference with protein-protein interactions mediated by coiled-coil motifs.Handb Exp Pharmacol. 2008;(186):461-82. doi: 10.1007/978-3-540-72843-6_19. Handb Exp Pharmacol. 2008. PMID: 18491064 Review.
Cited by
-
Human CCDC51 and yeast Mdm33 are functionally conserved mitochondrial inner membrane proteins that demarcate a subset of organelle fission events.bioRxiv [Preprint]. 2024 Mar 22:2024.03.21.586162. doi: 10.1101/2024.03.21.586162. bioRxiv. 2024. PMID: 38562768 Free PMC article. Preprint.
-
Evolutionary, comparative, and functional analyses of STATs and regulation of the JAK-STAT pathway in lumpfish upon bacterial and poly(I:C) exposure.Front Cell Infect Microbiol. 2023 Sep 22;13:1252744. doi: 10.3389/fcimb.2023.1252744. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37808912 Free PMC article.
-
Recognition of polymorphic Csd proteins determines sex in the honeybee.Sci Adv. 2023 Oct 6;9(40):eadg4239. doi: 10.1126/sciadv.adg4239. Epub 2023 Oct 4. Sci Adv. 2023. PMID: 37792946 Free PMC article.
-
Affinity-controlled capture and release of engineered monoclonal antibodies by macroporous dextran hydrogels using coiled-coil interactions.MAbs. 2023 Jan-Dec;15(1):2218951. doi: 10.1080/19420862.2023.2218951. MAbs. 2023. PMID: 37300397 Free PMC article.
-
Unconventional conservation reveals structure-function relationships in the synaptonemal complex.Elife. 2021 Nov 17;10:e72061. doi: 10.7554/eLife.72061. Elife. 2021. PMID: 34787570 Free PMC article.
References
-
- Abascal F, Posada D, Zardoya R. MtArt: a new model of amino acid replacement for Arthropoda. Mol Biol Evol. 2007;24:1–5. - PubMed
-
- Adachi J, Hasegawa M. Model of amino acid substitution in proteins encoded by mitochondrial DNA. J Mol Evol. 1996;42:459–468. - PubMed
-
- Adachi J, Waddell PJ, Martin W, Hasegawa M. Plastid genome phylogeny and a model of amino acid substitution for proteins encoded by chloroplast DNA. J Mol Evol. 2000;50:348–358. - PubMed
-
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


