Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway
- PMID: 19648118
- PMCID: PMC2785701
- DOI: 10.1074/jbc.M109.008177
Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway
Abstract
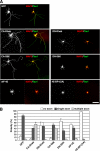
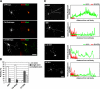
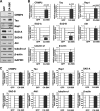
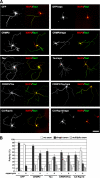
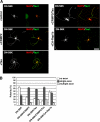
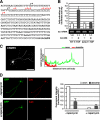
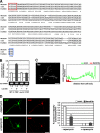
Mammalian target of rapamycin (mTOR) is an important regulator of neuronal development and functions. Although it was reported recently that mTOR signaling is critical for neuronal polarity, the underlying mechanism remains unclear. Here, we describe the molecular pathway of mTOR-dependent axon specification, in which the collapsing response mediator protein 2 (CRMP2) and Tau are major downstream targets. The activity of mTOR effector 70-kDa ribosomal protein S6 kinase (p70S6K) specifically increases in the axon during neuronal polarity formation. The mTOR inhibitor rapamycin suppresses the translation of some neuronal polarity proteins, including CRMP2 and Tau, thereby inhibiting axon formation. In contrast, constitutively active p70S6K up-regulates the translation of these molecules, thus inducing multiple axons. Exogenous CRMP2 and Tau facilitate axon formation, even in the presence of rapamycin. In the 5'-untranslated region of Tau and CRMP2 mRNAs, we identified a 5'-terminal oligopyrimidine tract, which mediates mTOR-governed protein synthesis. The 5'-terminal oligopyrimidine tract sequences of CRMP2 and Tau mRNAs strongly contribute to the up-regulation of their translation in the axon in response to the axonal activation of the mTOR-p70S6K pathway. Taken together, we conclude that the local translation of CRMP2 and Tau, regulated by mTOR-p70S6K, is critical for the specification of neuronal polarity.
Figures

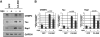
Similar articles
-
Docosahexaenoic Acid Promotes Axon Outgrowth by Translational Regulation of Tau and Collapsin Response Mediator Protein 2 Expression.J Biol Chem. 2016 Mar 4;291(10):4955-65. doi: 10.1074/jbc.M115.693499. Epub 2016 Jan 13. J Biol Chem. 2016. PMID: 26763232 Free PMC article.
-
Rapamycin Attenuated Zinc-Induced Tau Phosphorylation and Oxidative Stress in Rats: Involvement of Dual mTOR/p70S6K and Nrf2/HO-1 Pathways.Front Immunol. 2022 Feb 7;13:782434. doi: 10.3389/fimmu.2022.782434. eCollection 2022. Front Immunol. 2022. PMID: 35197970 Free PMC article.
-
Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways.Cancer Res. 2006 Jan 1;66(1):315-23. doi: 10.1158/0008-5472.CAN-05-2367. Cancer Res. 2006. PMID: 16397245
-
Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies.Cell Death Differ. 2009 Jan;16(1):46-56. doi: 10.1038/cdd.2008.110. Epub 2008 Jul 18. Cell Death Differ. 2009. PMID: 18636076 Review.
-
Increased CRMP2 phosphorylation is observed in Alzheimer's disease; does this tell us anything about disease development?Curr Alzheimer Res. 2009 Jun;6(3):269-78. doi: 10.2174/156720509788486572. Curr Alzheimer Res. 2009. PMID: 19519308 Review.
Cited by
-
Inhibition of ERK1/2 or CRMP2 Disrupts Alcohol Memory Reconsolidation and Prevents Relapse in Rats.Int J Mol Sci. 2024 May 17;25(10):5478. doi: 10.3390/ijms25105478. Int J Mol Sci. 2024. PMID: 38791516 Free PMC article.
-
Post-Translational Modifications in Tau and Their Roles in Alzheimer's Pathology.Curr Alzheimer Res. 2024;21(1):24-49. doi: 10.2174/0115672050301407240408033046. Curr Alzheimer Res. 2024. PMID: 38623984 Review.
-
The six brain-specific TAU isoforms and their role in Alzheimer's disease and related neurodegenerative dementia syndromes.Alzheimers Dement. 2024 May;20(5):3606-3628. doi: 10.1002/alz.13784. Epub 2024 Mar 31. Alzheimers Dement. 2024. PMID: 38556838 Free PMC article. Review.
-
The SMN-ribosome interplay: a new opportunity for Spinal Muscular Atrophy therapies.Biochem Soc Trans. 2024 Feb 28;52(1):465-479. doi: 10.1042/BST20231116. Biochem Soc Trans. 2024. PMID: 38391004 Free PMC article. Review.
-
The role of TSC1 and TSC2 proteins in neuronal axons.Mol Psychiatry. 2024 Apr;29(4):1165-1178. doi: 10.1038/s41380-023-02402-7. Epub 2024 Jan 11. Mol Psychiatry. 2024. PMID: 38212374 Review.
References
-
- Craig A. M., Banker G. (1994) Annu. Rev. Neurosci. 17, 267–310 - PubMed
-
- Arimura N., Kaibuchi K. (2007) Nat. Rev. Neurosci. 8, 194–205 - PubMed
-
- Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 - PubMed
-
- Swiech L., Perycz M., Malik A., Jaworski J. (2008) Biochim. Biophys. Acta 1784, 116–132 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


