MAM: more than just a housekeeper
- PMID: 19144519
- PMCID: PMC2750097
- DOI: 10.1016/j.tcb.2008.12.002
MAM: more than just a housekeeper
Abstract
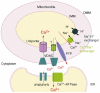
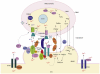
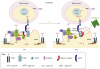
The physical association between the endoplasmic reticulum (ER) and mitochondria, which is known as the mitochondria-associated ER membrane (MAM), has important roles in various cellular 'housekeeping' functions including the non-vesicular transports of phospholipids. It has recently become clear that the MAM also enables highly efficient transmission of Ca(2+) from the ER to mitochondria to stimulate oxidative metabolism and, conversely, might enable the metabolically energized mitochondria to regulate the ER Ca(2+) homeostasis. Recent studies have shed light on molecular chaperones such as calnexin, calreticulin, ERp44, ERp57, grp75 and the sigma-1 receptor at the MAM, which regulate the association between the two organelles. The MAM thus integrates signal transduction with metabolic pathways to regulate the communication and functional interactions between the ER and mitochondrion.
Figures
Similar articles
-
Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM).Biochim Biophys Acta. 2010 Aug;1798(8):1465-73. doi: 10.1016/j.bbamem.2010.04.009. Epub 2010 Apr 27. Biochim Biophys Acta. 2010. PMID: 20430008 Free PMC article. Review.
-
Endoplasmic reticulum chaperones tweak the mitochondrial calcium rheostat to control metabolism and cell death.Cell Calcium. 2018 Mar;70:64-75. doi: 10.1016/j.ceca.2017.05.015. Epub 2017 May 31. Cell Calcium. 2018. PMID: 28619231 Review.
-
Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival.Cell. 2007 Nov 2;131(3):596-610. doi: 10.1016/j.cell.2007.08.036. Cell. 2007. PMID: 17981125
-
Role of Endoplasmic Reticulum-Mitochondria Communication in Type 2 Diabetes.Adv Exp Med Biol. 2017;997:171-186. doi: 10.1007/978-981-10-4567-7_13. Adv Exp Med Biol. 2017. PMID: 28815530 Review.
-
Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM).Biochim Biophys Acta. 2013 Jan;1833(1):213-24. doi: 10.1016/j.bbamcr.2012.04.013. Epub 2012 May 2. Biochim Biophys Acta. 2013. PMID: 22575682 Review.
Cited by
-
AKAP1/PKA-mediated GRP75 phosphorylation at mitochondria-associated endoplasmic reticulum membranes protects cancer cells against ferroptosis.Cell Death Differ. 2024 Nov 13. doi: 10.1038/s41418-024-01414-2. Online ahead of print. Cell Death Differ. 2024. PMID: 39537840
-
Inhibition of SGLT2 protects podocytes in diabetic kidney disease by rebalancing mitochondria-associated endoplasmic reticulum membranes.Cell Commun Signal. 2024 Nov 7;22(1):534. doi: 10.1186/s12964-024-01914-1. Cell Commun Signal. 2024. PMID: 39511548 Free PMC article.
-
Hoxa5 alleviates adipose tissue metabolic distortions in high-fat diet mice associated with a reduction in MERC.BMC Biol. 2024 Oct 29;22(1):247. doi: 10.1186/s12915-024-02047-0. BMC Biol. 2024. PMID: 39468535 Free PMC article.
-
ER-mitochondria contact sites regulate hepatic lipogenesis via Ip3r-Grp75-Vdac complex recruiting Seipin.Cell Commun Signal. 2024 Sep 30;22(1):464. doi: 10.1186/s12964-024-01829-x. Cell Commun Signal. 2024. PMID: 39350150 Free PMC article.
-
Mitochondrial Dysfunctions: Genetic and Cellular Implications Revealed by Various Model Organisms.Genes (Basel). 2024 Sep 1;15(9):1153. doi: 10.3390/genes15091153. Genes (Basel). 2024. PMID: 39336744 Free PMC article. Review.
References
-
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. - PubMed
-
- Varnai P, et al. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J. Biol. Chem. 2007;282:29678–29690. - PubMed
-
- Lysakowski A, et al. Dense-cored vesicles, smooth endoplasmic reticulum, and mitochondria are closely associated with non-specialized parts of plasma membrane of nerve terminals: implications for exocytosis and calcium buffering by intraterminal organelles. J. Comp. Neurol. 1999;403:378–390. - PubMed
-
- Malli R, et al. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J. Biol. Chem. 2003;278:10807–10815. - PubMed
-
- Vance JE. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog. Nucleic Acid Res. Mol. Biol. 2003;75:69–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous


