Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960
- PMID: 18833279
- PMCID: PMC3682493
- DOI: 10.1038/nature07390
Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960
Abstract
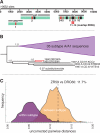
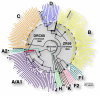
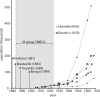
Human immunodeficiency virus type 1 (HIV-1) sequences that pre-date the recognition of AIDS are critical to defining the time of origin and the timescale of virus evolution. A viral sequence from 1959 (ZR59) is the oldest known HIV-1 infection. Other historically documented sequences, important calibration points to convert evolutionary distance into time, are lacking, however; ZR59 is the only one sampled before 1976. Here we report the amplification and characterization of viral sequences from a Bouin's-fixed paraffin-embedded lymph node biopsy specimen obtained in 1960 from an adult female in Léopoldville, Belgian Congo (now Kinshasa, Democratic Republic of the Congo (DRC)), and we use them to conduct the first comparative evolutionary genetic study of early pre-AIDS epidemic HIV-1 group M viruses. Phylogenetic analyses position this viral sequence (DRC60) closest to the ancestral node of subtype A (excluding A2). Relaxed molecular clock analyses incorporating DRC60 and ZR59 date the most recent common ancestor of the M group to near the beginning of the twentieth century. The sizeable genetic distance between DRC60 and ZR59 directly demonstrates that diversification of HIV-1 in west-central Africa occurred long before the recognized AIDS pandemic. The recovery of viral gene sequences from decades-old paraffin-embedded tissues opens the door to a detailed palaeovirological investigation of the evolutionary history of HIV-1 that is not accessible by other methods.
Figures
Comment in
-
AIDS: prehistory of HIV-1.Nature. 2008 Oct 2;455(7213):605-6. doi: 10.1038/455605a. Nature. 2008. PMID: 18833267 No abstract available.
Similar articles
-
An African HIV-1 sequence from 1959 and implications for the origin of the epidemic.Nature. 1998 Feb 5;391(6667):594-7. doi: 10.1038/35400. Nature. 1998. PMID: 9468138
-
A near full-length HIV-1 genome from 1966 recovered from formalin-fixed paraffin-embedded tissue.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12222-12229. doi: 10.1073/pnas.1913682117. Epub 2020 May 19. Proc Natl Acad Sci U S A. 2020. PMID: 32430331 Free PMC article.
-
Unprecedented degree of human immunodeficiency virus type 1 (HIV-1) group M genetic diversity in the Democratic Republic of Congo suggests that the HIV-1 pandemic originated in Central Africa.J Virol. 2000 Nov;74(22):10498-507. doi: 10.1128/jvi.74.22.10498-10507.2000. J Virol. 2000. PMID: 11044094 Free PMC article.
-
Using human immunodeficiency virus type 1 sequences to infer historical features of the acquired immune deficiency syndrome epidemic and human immunodeficiency virus evolution.Philos Trans R Soc Lond B Biol Sci. 2001 Jun 29;356(1410):855-66. doi: 10.1098/rstb.2001.0859. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11405933 Free PMC article. Review.
-
Elucidation of Early Evolution of HIV-1 Group M in the Congo Basin Using Computational Methods.Genes (Basel). 2021 Apr 2;12(4):517. doi: 10.3390/genes12040517. Genes (Basel). 2021. PMID: 33918115 Free PMC article. Review.
Cited by
-
Classification of a Massive Number of Viral Genomes and Estimation of Time of Most Recent Common Ancestor (tMRCA) of SARS-CoV-2 Using Phylodynamic Analysis.Bio Protoc. 2024 Mar 20;14(6):e4955. doi: 10.21769/BioProtoc.4955. eCollection 2024 Mar 20. Bio Protoc. 2024. PMID: 38835995 Free PMC article.
-
HIV co-infection is associated with reduced Mycobacterium tuberculosis transmissibility in sub-Saharan Africa.PLoS Pathog. 2024 May 2;20(5):e1011675. doi: 10.1371/journal.ppat.1011675. eCollection 2024 May. PLoS Pathog. 2024. PMID: 38696531 Free PMC article.
-
The Coronavirus Calendar (CoronaCal): a simplified SARS-CoV-2 test system for sampling and retrospective analysis.Front Epidemiol. 2023 May 23;3:1146006. doi: 10.3389/fepid.2023.1146006. eCollection 2023. Front Epidemiol. 2023. PMID: 38455914 Free PMC article.
-
Distinct genetic clusters in HIV-1 CRF01_AE-infected patients induced variable degrees of CD4+ T-cell loss.mBio. 2024 Mar 13;15(3):e0334923. doi: 10.1128/mbio.03349-23. Epub 2024 Feb 22. mBio. 2024. PMID: 38385695 Free PMC article.
-
HIV-1 Gag, Pol, and Env diversified with limited adaptation since the 1980s.mBio. 2024 Mar 13;15(3):e0174923. doi: 10.1128/mbio.01749-23. Epub 2024 Feb 8. mBio. 2024. PMID: 38329340 Free PMC article.
References
-
- Zhu TF, et al. An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. Nature. 1998;391:594–597. - PubMed
-
- Korber B, et al. Timing the ancestor of the HIV-1 pandemic strains. Science. 2000;288:1789–1796. - PubMed
-
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases


