Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases
- PMID: 18587387
- PMCID: PMC3422503
- DOI: 10.1038/nbt1410
Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases
Abstract
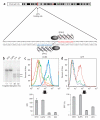
Homozygosity for the naturally occurring Delta32 deletion in the HIV co-receptor CCR5 confers resistance to HIV-1 infection. We generated an HIV-resistant genotype de novo using engineered zinc-finger nucleases (ZFNs) to disrupt endogenous CCR5. Transient expression of CCR5 ZFNs permanently and specifically disrupted approximately 50% of CCR5 alleles in a pool of primary human CD4(+) T cells. Genetic disruption of CCR5 provided robust, stable and heritable protection against HIV-1 infection in vitro and in vivo in a NOG model of HIV infection. HIV-1-infected mice engrafted with ZFN-modified CD4(+) T cells had lower viral loads and higher CD4(+) T-cell counts than mice engrafted with wild-type CD4(+) T cells, consistent with the potential to reconstitute immune function in individuals with HIV/AIDS by maintenance of an HIV-resistant CD4(+) T-cell population. Thus adoptive transfer of ex vivo expanded CCR5 ZFN-modified autologous CD4(+) T cells in HIV patients is an attractive approach for the treatment of HIV-1 infection.
Figures

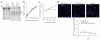

Similar articles
-
Engineering HIV-resistant human CD4+ T cells with CXCR4-specific zinc-finger nucleases.PLoS Pathog. 2011 Apr;7(4):e1002020. doi: 10.1371/journal.ppat.1002020. Epub 2011 Apr 14. PLoS Pathog. 2011. PMID: 21533216 Free PMC article.
-
Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo.Nat Biotechnol. 2010 Aug;28(8):839-47. doi: 10.1038/nbt.1663. Epub 2010 Jul 2. Nat Biotechnol. 2010. PMID: 20601939 Free PMC article.
-
Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5.Hum Gene Ther. 2013 Mar;24(3):245-58. doi: 10.1089/hum.2012.172. Epub 2013 Mar 6. Hum Gene Ther. 2013. PMID: 23360514 Free PMC article.
-
Chemokine receptor 5 knockout strategies.Curr Opin HIV AIDS. 2011 Jan;6(1):74-9. doi: 10.1097/COH.0b013e32834122d7. Curr Opin HIV AIDS. 2011. PMID: 21242897 Free PMC article. Review.
-
Pre-clinical modeling of CCR5 knockout in human hematopoietic stem cells by zinc finger nucleases using humanized mice.J Infect Dis. 2013 Nov;208 Suppl 2(Suppl 2):S160-4. doi: 10.1093/infdis/jit382. J Infect Dis. 2013. PMID: 24151324 Free PMC article. Review.
Cited by
-
Interventions during Early Infection: Opening a Window for an HIV Cure?Viruses. 2024 Oct 9;16(10):1588. doi: 10.3390/v16101588. Viruses. 2024. PMID: 39459922 Free PMC article. Review.
-
Cell-Penetrating Peptides and CRISPR-Cas9: A Combined Strategy for Human Genetic Disease Therapy.Hum Gene Ther. 2024 Oct;35(19-20):781-797. doi: 10.1089/hum.2024.020. Hum Gene Ther. 2024. PMID: 39276086 Review.
-
Exploring the potential of cell-derived vesicles for transient delivery of gene editing payloads.Adv Drug Deliv Rev. 2024 Aug;211:115346. doi: 10.1016/j.addr.2024.115346. Epub 2024 Jun 6. Adv Drug Deliv Rev. 2024. PMID: 38849005 Free PMC article. Review.
-
A simultaneous knockout knockin genome editing strategy in HSPCs potently inhibits CCR5- and CXCR4-tropic HIV-1 infection.Cell Stem Cell. 2024 Apr 4;31(4):499-518.e6. doi: 10.1016/j.stem.2024.03.002. Cell Stem Cell. 2024. PMID: 38579682
-
Preclinical toxicity analyses of lentiviral vectors expressing the HIV-1 LTR-specific designer-recombinase Brec1.PLoS One. 2024 Mar 8;19(3):e0298542. doi: 10.1371/journal.pone.0298542. eCollection 2024. PLoS One. 2024. PMID: 38457474 Free PMC article.
References
-
- Deng HK, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. - PubMed
-
- Alkhatib G, et al. Cc Ckrs: A Rantes, Mip-1 Alpha, Mip-1 Beta Receptor As A Fusion Cofactor for Macrophage-Tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Liu R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. - PubMed
-
- Samson M, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. - PubMed
-
- Huang YX, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996;2:1240–1243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials


