Characterization of the carboxysomal carbonic anhydrase CsoSCA from Halothiobacillus neapolitanus
- PMID: 17012396
- PMCID: PMC1698195
- DOI: 10.1128/JB.00990-06
Characterization of the carboxysomal carbonic anhydrase CsoSCA from Halothiobacillus neapolitanus
Abstract
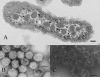
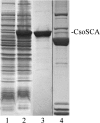
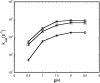
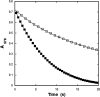
In cyanobacteria and many chemolithotrophic bacteria, the CO(2)-fixing enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) is sequestered into polyhedral protein bodies called carboxysomes. The carboxysome is believed to function as a microcompartment that enhances the catalytic efficacy of RubisCO by providing the enzyme with its substrate, CO(2), through the action of the shell protein CsoSCA, which is a novel carbonic anhydrase. In the work reported here, the biochemical properties of purified, recombinant CsoSCA were studied, and the catalytic characteristics of the carbonic anhydrase for the CO(2) hydration and bicarbonate dehydration reactions were compared with those of intact and ruptured carboxysomes. The low apparent catalytic rates measured for CsoSCA in intact carboxysomes suggest that the protein shell acts as a barrier for the CO(2) that has been produced by CsoSCA through directional dehydration of cytoplasmic bicarbonate. This CO(2) trap provides the sequestered RubisCO with ample substrate for efficient fixation and constitutes a means by which microcompartmentalization enhances the catalytic efficiency of this enzyme.
Figures
Similar articles
-
Identification of a carbonic anhydrase-Rubisco complex within the alpha-carboxysome.Proc Natl Acad Sci U S A. 2023 Oct 24;120(43):e2308600120. doi: 10.1073/pnas.2308600120. Epub 2023 Oct 20. Proc Natl Acad Sci U S A. 2023. PMID: 37862384 Free PMC article.
-
CO2 fixation kinetics of Halothiobacillus neapolitanus mutant carboxysomes lacking carbonic anhydrase suggest the shell acts as a diffusional barrier for CO2.J Biol Chem. 2008 Apr 18;283(16):10377-84. doi: 10.1074/jbc.M709285200. Epub 2008 Feb 7. J Biol Chem. 2008. PMID: 18258595
-
The structure of beta-carbonic anhydrase from the carboxysomal shell reveals a distinct subclass with one active site for the price of two.J Biol Chem. 2006 Mar 17;281(11):7546-55. doi: 10.1074/jbc.M510464200. Epub 2006 Jan 10. J Biol Chem. 2006. PMID: 16407248
-
Carboxysomal carbonic anhydrases.Subcell Biochem. 2014;75:89-103. doi: 10.1007/978-94-007-7359-2_6. Subcell Biochem. 2014. PMID: 24146376 Review.
-
Carboxysomal carbonic anhydrases: Structure and role in microbial CO2 fixation.Biochim Biophys Acta. 2010 Feb;1804(2):382-92. doi: 10.1016/j.bbapap.2009.09.026. Epub 2009 Oct 8. Biochim Biophys Acta. 2010. PMID: 19818881 Review.
Cited by
-
A new type of carboxysomal carbonic anhydrase in sulfur chemolithoautotrophs from alkaline environments.Appl Environ Microbiol. 2024 Sep 18;90(9):e0107524. doi: 10.1128/aem.01075-24. Epub 2024 Aug 23. Appl Environ Microbiol. 2024. PMID: 39177330
-
Cyanobacterial α-carboxysome carbonic anhydrase is allosterically regulated by the Rubisco substrate RuBP.Sci Adv. 2024 May 10;10(19):eadk7283. doi: 10.1126/sciadv.adk7283. Epub 2024 May 10. Sci Adv. 2024. PMID: 38728392 Free PMC article.
-
Widespread dissolved inorganic carbon-modifying toolkits in genomes of autotrophic Bacteria and Archaea and how they are likely to bridge supply from the environment to demand by autotrophic pathways.Appl Environ Microbiol. 2024 Feb 21;90(2):e0155723. doi: 10.1128/aem.01557-23. Epub 2024 Feb 1. Appl Environ Microbiol. 2024. PMID: 38299815 Free PMC article. Review.
-
α-Carboxysome Size Is Controlled by the Disordered Scaffold Protein CsoS2.Biochemistry. 2024 Jan 16;63(2):219-229. doi: 10.1021/acs.biochem.3c00403. Epub 2023 Dec 12. Biochemistry. 2024. PMID: 38085650 Free PMC article.
-
Identification of a carbonic anhydrase-Rubisco complex within the alpha-carboxysome.Proc Natl Acad Sci U S A. 2023 Oct 24;120(43):e2308600120. doi: 10.1073/pnas.2308600120. Epub 2023 Oct 20. Proc Natl Acad Sci U S A. 2023. PMID: 37862384 Free PMC article.
References
-
- Alber, B. E., C. M. Colangelo, J. Dong, C. M. V. Stalhandske, T. T. Baird, C. Tu, A. Fierke, D. N. Silverman, R. A. Scott, and J. G. Ferry. 1999. Kinetic and spectroscopic characterization of the gamma carbonic anhydrase from the methanoarchaeon Methanosarcina thermophila. Biochemistry 38:13119-13128. - PubMed
-
- Armstrong, J. M., D. V. Myers, J. A. Verpoorte, and J. T. Edsall. 1966. Purification and properties of human erythrocyte carbonic anhydrases. J. Biol. Chem. 241:5137-5149. - PubMed
-
- Badger, M. R. 2003. The roles of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth. Res. 77:83-94. - PubMed
-
- Badger, M. R., D. Hanson, and G. D. Price. 2002. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct. Plant Biol. 29:161-173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources


