Rotavirus-specific T-cell responses in young prospectively followed-up children
- PMID: 15196259
- PMCID: PMC1809077
- DOI: 10.1111/j.1365-2249.2004.02509.x
Rotavirus-specific T-cell responses in young prospectively followed-up children
Abstract
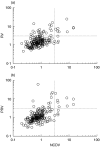
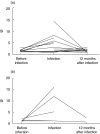
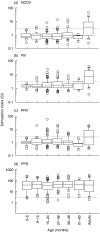
Rotavirus is a major cause of gastroenteritis in young children. Antibodies seem to protect against rotavirus infection but cell-mediated immune responses are probably also important for protection. We evaluated the development of T-cell responses to rotavirus in follow-up samples from 20 healthy children with an increased genetic risk for type 1 diabetes. Blood samples from 16 healthy adults were also available for the study. T-cell proliferation was analysed at 3-6 month intervals from the age of 3 months to the age of 4-5 years using the Wa strain of human rotavirus and the NCDV strain of bovine rotavirus as antigens. IgG and IgA antibodies to rotavirus were studied from simultaneously drawn plasma samples with EIA method using NCDV as an antigen. A total of 24 infections were revealed by antibody analysis. Sixteen children showed diagnostic increases in both IgG and IgA antibodies to rotavirus, while 5 children showed increases in IgA antibodies only and 3 in IgG only. Antibody rises were accompanied by T-cell responses to rotavirus (SI > 3) in 9 of the 24 cases. T-cell responses to purified or lysed human rotavirus were stronger after a rise in rotavirus antibodies than the responses before infection (P = 0.017 and 0.027, respectively). There was a correlation between T-cell responses to purified and lysed human rotavirus and NCDV. Strong T-cell responses to rotavirus were transient and the ability to respond usually disappeared in one year, but in all adults T-cell responses to rotavirus were strong implicating that several infections are needed to develop consistent, strong T-cell responsiveness.
Figures
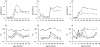
Similar articles
-
Systemic and intestinal antibody responses to NSP4 enterotoxin of Wa human rotavirus in a gnotobiotic pig model of human rotavirus disease.J Med Virol. 2002 Sep;68(1):119-28. doi: 10.1002/jmv.10178. J Med Virol. 2002. PMID: 12210439
-
Rotavirus-specific T cell responses and cytokine mRNA expression in children with diabetes-associated autoantibodies and type 1 diabetes.Clin Exp Immunol. 2006 Aug;145(2):261-70. doi: 10.1111/j.1365-2249.2006.03146.x. Clin Exp Immunol. 2006. PMID: 16879245 Free PMC article.
-
Memory T-cell response to rotavirus detected with a gamma interferon enzyme-linked immunospot assay.J Virol. 2005 May;79(9):5684-94. doi: 10.1128/JVI.79.9.5684-5694.2005. J Virol. 2005. PMID: 15827183 Free PMC article.
-
Natural immunity to rotavirus infection in children.Indian J Biochem Biophys. 2008 Aug;45(4):219-28. Indian J Biochem Biophys. 2008. PMID: 18788471 Review.
-
Human immunity to rotavirus.J Med Microbiol. 1995 Dec;43(6):397-404. doi: 10.1099/00222615-43-6-397. J Med Microbiol. 1995. PMID: 7473672 Review.
Cited by
-
Possible association of rotavirus IgG with cytokine expression levels and dyslipidemia in rotavirus-infected type 1 diabetic children.Mol Biol Rep. 2022 Aug;49(8):7587-7599. doi: 10.1007/s11033-022-07573-0. Epub 2022 Jun 22. Mol Biol Rep. 2022. PMID: 35733062 Free PMC article.
-
Rotaviruses: From Pathogenesis to Disease Control-A Critical Review.Viruses. 2022 Apr 22;14(5):875. doi: 10.3390/v14050875. Viruses. 2022. PMID: 35632617 Free PMC article. Review.
-
T-Cell Responses after Rotavirus Infection or Vaccination in Children: A Systematic Review.Viruses. 2022 Feb 23;14(3):459. doi: 10.3390/v14030459. Viruses. 2022. PMID: 35336866 Free PMC article. Review.
-
Serological Humoral Immunity Following Natural Infection of Children with High Burden Gastrointestinal Viruses.Viruses. 2021 Oct 9;13(10):2033. doi: 10.3390/v13102033. Viruses. 2021. PMID: 34696463 Free PMC article. Review.
-
Surveillance data confirm multiyear predictions of rotavirus dynamics in New York City.Sci Adv. 2020 Feb 26;6(9):eaax0586. doi: 10.1126/sciadv.aax0586. eCollection 2020 Feb. Sci Adv. 2020. PMID: 32133392 Free PMC article.
References
-
- Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Philadelphia: Lippincott. Williams & Wilkins; 2001. pp. 1787–833.
-
- Kapikian AZ, Wyatt RG, Levine MM, et al. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983;147:95–106. - PubMed
-
- Ward RL, Bernstein DI, Shukla R, et al. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989;159:79–88. - PubMed
-
- Clemens JD, Ward RL, Rao MR, et al. Seroepidemiologic evaluation of antibodies to rotavirus as correlates of the risk of clinically significant rotavirus diarrhea in rural Bangladesh. J Infect Dis. 1992;165:161–5. - PubMed
-
- Ward RL, Bernstein DI. Protection against rotavirus disease after natural rotavirus infection. US Rotavirus Vaccine Efficacy Group. J Infect Dis. 1994;169:900–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


