The functional organization of mitochondrial genomes in human cells
- PMID: 15157274
- PMCID: PMC425603
- DOI: 10.1186/1741-7007-2-9
The functional organization of mitochondrial genomes in human cells
Abstract
Background: We analyzed the organization and function of mitochondrial DNA in a stable human cell line (ECV304, which is also known as T-24) containing mitochondria tagged with the yellow fluorescent protein.
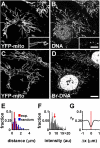
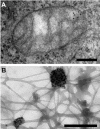
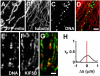
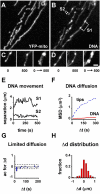
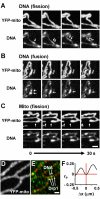
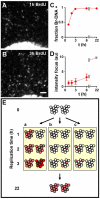
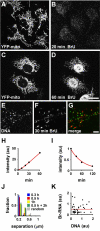
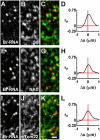
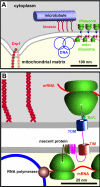
Results: Mitochondrial DNA is organized in approximately 475 discrete foci containing 6-10 genomes. These foci (nucleoids) are tethered directly or indirectly through mitochondrial membranes to kinesin, marked by KIF5B, and microtubules in the surrounding cytoplasm. In living cells, foci have an apparent diffusion constant of 1.1 x 10(-3) microm2/s, and mitochondria always split next to a focus to distribute all DNA to one daughter. The kinetics of replication and transcription (monitored by immunolabelling after incorporating bromodeoxyuridine or bromouridine) reveal that each genome replicates independently of others in a focus, and that newly-made RNA remains in a focus (residence half-time approximately 43 min) long after it has been made. This mitochondrial RNA colocalizes with components of the cytoplasmic machinery that makes and imports nuclear-encoded proteins - that is, a ribosomal protein (S6), a nascent peptide associated protein (NAC), and the translocase in the outer membrane (Tom22).
Conclusions: The results suggest that clusters of mitochondrial genomes organize the translation machineries on both sides of the mitochondrial membranes. Then, proteins encoded by the nuclear genome and destined for the mitochondria will be made close to mitochondrial-encoded proteins so that they can be assembled efficiently into mitochondrial complexes.
Figures
Similar articles
-
Organization and Regulation of Mitochondrial Protein Synthesis.Annu Rev Biochem. 2016 Jun 2;85:77-101. doi: 10.1146/annurev-biochem-060815-014334. Epub 2016 Jan 18. Annu Rev Biochem. 2016. PMID: 26789594 Review.
-
Effects of Fcj1-Mos1 and mitochondrial division on aggregation of mitochondrial DNA nucleoids and organelle morphology.Mol Biol Cell. 2013 Jun;24(12):1842-51. doi: 10.1091/mbc.E13-03-0125. Epub 2013 Apr 24. Mol Biol Cell. 2013. PMID: 23615445 Free PMC article.
-
The plant mitochondrial genome: dynamics and maintenance.Biochimie. 2014 May;100:107-20. doi: 10.1016/j.biochi.2013.09.016. Epub 2013 Sep 26. Biochimie. 2014. PMID: 24075874 Review.
-
Ribosome recycling defects modify the balance between the synthesis and assembly of specific subunits of the oxidative phosphorylation complexes in yeast mitochondria.Nucleic Acids Res. 2016 Jul 8;44(12):5785-97. doi: 10.1093/nar/gkw490. Epub 2016 Jun 1. Nucleic Acids Res. 2016. PMID: 27257059 Free PMC article.
-
Nuclear genes and mitochondrial translation: a new class of genetic disease.Trends Genet. 2005 Jun;21(6):312-4. doi: 10.1016/j.tig.2005.04.003. Trends Genet. 2005. PMID: 15922826 Review.
Cited by
-
Elevated serum mtDNA in COVID-19 patients is linked to SARS-CoV-2 envelope protein targeting mitochondrial VDAC1, inducing apoptosis and mtDNA release.Apoptosis. 2024 Dec;29(11-12):2025-2046. doi: 10.1007/s10495-024-02025-5. Epub 2024 Oct 7. Apoptosis. 2024. PMID: 39375263 Free PMC article.
-
Mitochondrial double-stranded RNA homeostasis depends on cell-cycle progression.Life Sci Alliance. 2024 Aug 29;7(11):e202402764. doi: 10.26508/lsa.202402764. Print 2024 Nov. Life Sci Alliance. 2024. PMID: 39209534 Free PMC article.
-
Real-time assessment of mitochondrial DNA heteroplasmy dynamics at the single-cell level.EMBO J. 2024 Nov;43(22):5340-5359. doi: 10.1038/s44318-024-00183-5. Epub 2024 Aug 5. EMBO J. 2024. PMID: 39103491 Free PMC article.
-
A multiscale model of the role of microenvironmental factors in cell segregation and heterogeneity in breast cancer development.PLoS Comput Biol. 2023 Nov 22;19(11):e1011673. doi: 10.1371/journal.pcbi.1011673. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 37992135 Free PMC article.
-
Unprecedented enantio-selective live-cell mitochondrial DNA super-resolution imaging and photo-sensitizing by the chiral ruthenium polypyridyl DNA "light-switch".Nucleic Acids Res. 2023 Dec 11;51(22):11981-11998. doi: 10.1093/nar/gkad799. Nucleic Acids Res. 2023. PMID: 37933856 Free PMC article.
References
-
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. - PubMed
-
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


