Abstract
Background
Oral cancer is an important global healthcare problem, its incidence is increasing and late‐stage presentation is common. Screening programmes have been introduced for a number of major cancers and have proved effective in their early detection. Given the high morbidity and mortality rates associated with oral cancer, there is a need to determine the effectiveness of a screening programme for this disease, either as a targeted, opportunistic or population‐based measure. Evidence exists from modelled data that a visual oral examination of high‐risk individuals may be a cost‐effective screening strategy and the development and use of adjunctive aids and biomarkers is becoming increasingly common.
Objectives
To assess the effectiveness of current screening methods in decreasing oral cancer mortality.
Search methods
We searched the following electronic databases: the Cochrane Oral Health Group's Trials Register (to 22 July 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 6), MEDLINE via OVID (1946 to 22 July 2013), EMBASE via OVID (1980 to 22 July 2013) and CANCERLIT via PubMed (1950 to 22 July 2013). There were no restrictions on language in the search of the electronic databases.
Selection criteria
Randomised controlled trials (RCTs) of screening for oral cancer or potentially malignant disorders using visual examination, toluidine blue, fluorescence imaging or brush biopsy.
Data collection and analysis
Two review authors screened the results of the searches against inclusion criteria, extracted data and assessed risk of bias independently and in duplicate. We used mean differences (MDs) and 95% confidence intervals (CIs) for continuous data and risk ratios (RRs) with 95% CIs for dichotomous data. Meta‐analyses would have been undertaken using a random‐effects model if the number of studies had exceeded a minimum of three. Study authors were contacted where possible and where deemed necessary for missing information.
Main results
A total of 3239 citations were identified through the searches. Only one RCT, with 15‐year follow‐up met the inclusion criteria (n = 13 clusters: 191,873 participants). There was no statistically significant difference in the oral cancer mortality rates for the screened group (15.4/100,000 person‐years) and the control group (17.1/100,000 person‐years), with a RR of 0.88 (95% CI 0.69 to 1.12). A 24% reduction in mortality was reported between the screening group (30/100,000 person‐years) and the control group (39.0/100,000) for high‐risk individuals who used tobacco or alcohol or both, which was statistically significant (RR 0.76; 95% CI 0.60 to 0.97). No statistically significant differences were found for incidence rates. A statistically significant reduction in the number of individuals diagnosed with stage III or worse oral cancer was found for those in the screening group (RR 0.81; 95% CI 0.70 to 0.93). No harms were reported. The study was assessed as at high risk of bias.
Authors' conclusions
There is evidence that a visual examination as part of a population‐based screening programme reduces the mortality rate of oral cancer in high‐risk individuals. In addition, there is a stage shift and improvement in survival rates across the population as a whole. However, the evidence is limited to one study, which has a high risk of bias and did not account for the effect of cluster randomisation in the analysis. There was no evidence to support the use of adjunctive technologies like toluidine blue, brush biopsy or fluorescence imaging as a screening tool to reduce oral cancer mortality. Further RCTs are recommended to assess the efficacy and cost‐effectiveness of a visual examination as part of a population‐based screening programme in low, middle and high‐income countries.
Plain language summary
Screening programmes for the early detection and prevention of oral cancer
Review question
This review, carried out by authors of the Cochrane Oral Health Group, was conducted to investigate the effectiveness of current screening programmes in detecting oral cancer at an early stage and whether or not they can assist in decreasing deaths due to oral cancer.
Background
Oral cancer is increasing worldwide and it is the sixth most common cancer overall. The highest rates of oral cancer occur in the most disadvantaged sections of the population. Important risk factors in the development of the disease are tobacco, alcohol, age, gender and sunlight although a role for candida (which causes thrush) and the human papillomavirus (which causes warts) has also been documented. People who are heavy drinkers and also smoke have 38 times the risk of developing oral cancer compared with people who do neither. These factors are considered to be especially important in the development of the disease in young people, a group experiencing an increasing incidence of the disease, particularly in countries with a high incidence of it.
Geographic variation in the occurrence of oral cancer around the world is wide. For example it is the most common cancer for men in India, Sri Lanka and Pakistan and 30% of all new cases of cancer in these countries is oral cancer whereas only 3% of new cases of cancer in the United Kingdom are oral cancer.
When people first seek medical help, their oral cancer is usually at a late or advanced stage and the effects of the condition as well as the treatment for it can be extremely debilitating. Death rates from oral cancer and the negative effects of the disease are high and increasing rather than declining as for other cancers such as breast and colon.
Prevention screening programmes for other cancers have proven to be effective in early detection. However, whilst there maybe advantages to screening there are disadvantages because screening has the potential to produce either false positive or false negative results. Screening can be targeted at high‐risk groups, it can be opportunistic, for example when people attend health services for other reasons, or can be done by looking at statistics across the population as a whole.
The aim of preventive screening for early detection of oral cancer is to screen individuals for pre‐cancerous conditions which are lesions such as leukoplakia. The most common screening method is visual inspection by a clinician but other techniques include the use of a special blue dye, the use of imaging techniques and measuring biochemical changes to normal calls.
Study characteristics
The evidence on which this review is based is up to date as of 22 July 2013. The only study included was based in rural areas of the city of Trivandrum in Kerala, India. The study included 191,873 apparently healthy adults aged 35 years or older living in 13 clusters with an average of 14,759 participants in each cluster. Screening took place in seven clusters (96,517 participants) and six clusters acted as a control (95,356 participants). Participants were excluded if they were bedridden, if they had open tuberculosis, other debilitating diseases or were already suffering from oral cancer.
Healthcare workers trained in the detection of oral lesions undertook the screening of participants and the social history of participants including use of paan, tobacco, alcohol and dietary supplements was recorded.
Key results
The review found that overall there is not enough evidence to decide whether screening by visual inspection reduces the death rate for oral cancer and there is no evidence for other screening methods. However, there is some evidence that it might help reduce death rates in patients who use tobacco and alcohol although the only included study may be affected by bias.
Quality of evidence
The evidence presented is of low quality and limited to one study assessed as at high risk of bias.
Background
Description of the condition
Oral cancer is the sixth most common cancer globally and represents a group of conditions with a range of sites and a varied aetiology. Its annual estimated incidence is approximately 275,000, but unlike many other cancers, its incidence is increasing (Warnakulasuriya 2009). There is a wide geographic variation in the incidence of the disease with two‐thirds of the burden born by low‐income and middle‐income countries from South and South‐East Asia, Latin America and Eastern Europe. However, the incidence continues to rise in the West (IARC 2010) and the age standardised incidence of oral cancer in Western Europe has steadily increased over the past two decades (Boyle 2005). Within the European Union countries, the highest male incidence rates are found in France and Hungary, whilst the lowest rates are found in Greece and Cyprus (IARC 2010). India, Sri Lanka and Pakistan have the highest levels of disease, making it the most common cancer for men in these countries and accounts for up to 30% of all new cases of cancer compared to 3% in the United Kingdom (UK) and 6% in France (Cancer Research UK). The age‐adjusted incidence rate from these countries cancer registries range from 3.4 to 13.8 per 100,000 (Ministry of Health 2005; Warnakulasuriya 2009). The incidence of oral cancer for men in Brazil is second only to France and India with an estimated crude rate of 11 per 100,000. In the UK, the incidence of oral cancer is increasing (Conway 2006; Doobaree 2009) and 6236 cases of oral cancer were diagnosed in 2009 (Cancer Research UK). This represents a doubling of the number of cases seen in 1989 and represents a year on year increase of approximately 2.7% per year (Warnakulasuriya 2009). The incidence of oral cancer is strongly associated with social and economic deprivation (Scully 2009; Conway 2010a), with the highest rates occurring in the most disadvantaged sections of the population. Across Europe, inequalities tend to be observed among men, particularly in the UK and Eastern Europe (Conway 2010b).
Important risk factors in the development of the disease are tobacco, betel quid, alcohol, age, gender and sunlight. More recently, a role for candida and the human papillomavirus (HPV) has been documented (Scully 2009). Epidemiological evidence from US populations indicates a strong association between HPV and oropharyngeal cancers (Cleveland 2011). Incidence of HPV globally is unknown but thought to be rising, but estimates from the United States of America (USA) show a substantial increase of HPV‐positive oropharyngeal cancers, rising by 225% in the period between 1984 and 2004 (Sanders 2011). Increased consumption of alcohol has been implicated in the increasing incidence of the disease in the UK (Hindle 2000) at a time when tobacco use is falling (Ogden 2005), although the precise mechanism remains unclear (Ogden 1998). Heavy drinkers and smokers have 38 times the risk of developing oral cancer compared to abstainers (Blot 1988). This is thought to be due to acetaldehyde, the first metabolite of alcohol, which is classified as a Group 1 carcinogen and is also present in tobacco (Salaspuro 2011). Historically, the risk of developing oral cancer increased with age, however, the age group with the highest incidence (26.8%) in the USA between 2003 and 2007 was between 55 and 64 years of age (SEER 2010). In contrast, many patients from high‐incidence countries are below the age of 40 years of age (Warnakulasuriya 2009).
Of equal concern to the increasing incidence, is the lack of any change in the age‐standardised mortality rates, despite advances in surgical and management techniques. This is unlike the falling rates for cancer of the breast and colon (Cancer Research UK). The five‐year survival rates for oral cancer for most countries is approximately 50% (Warnakulasuriya 2009). These have been estimated at 3 to 4 per 100,000 men and 1.5 to 2.0 per 100,000 for women respectively (Warnakulasuriya 2009). Mortality rates from oral cancer have also increased in certain European countries (La Vecchia 2004). The most important determinant factor in cancer survival is diagnostic delay (Onizawa 2003; McLeod 2005), as over 60% of patients present with stage III and IV disease (Lingen 2008), meaning that their management is complex and multidisciplinary. The stage at diagnosis significantly affects five‐year survival, with survival rates approaching 80% for stage I disease, whilst dropping significantly for stage IV disease (Rusthoven 2010). In addition, the morbidity associated with surgery is high, the rate of second primary tumours is greater than any other type of cancer (3% to 7% per annum) (Day 1992) and is more often the cause of death (Lippman 1989).
Description of the intervention
Prevention strategies are important to meet the World Health Organization's (WHO) resolution to incorporate oral cancer into national cancer control programs (Petersen 2009). Although it is important to continue to clarify the public health message and promote primary prevention, determining the feasibility of a national screening programme is an important step in the prevention of the disease. The National Screening Committee define screening as "a process of identifying apparently healthy people who may be at increased risk of a disease or condition" (NSC 2010). Screening can be undertaken across the whole population, opportunistically, when individuals are attending for some other purpose, or selectively, where high‐risk groups are targeted. Programmes for major cancers, such as breast, cervical and bowel cancer have effectively improved the mortality rates and helped to decrease the incidence of these cancers (Gøtzsche 2006; Hewitson 2007). However, it is important to consider both the harms and benefits of any screening programmes. For example, screening for breast cancer has recently been associated with over‐diagnosis and unnecessary treatment causing further physical and psychological harms (Gøtzsche 2013) and so any future programme should always balance these considerations.
How the intervention might work
Screening is predicated on the idea that malignancy is preceded by clinically evident lesions, which if identified early and removed, can either prevent their malignant transformation or reduce their staging. The majority of oral carcinomas are preceded by visible lesions, known as potentially malignant disorders (PMDs) (Warnakulasuriya 2007; van der Waal 2009) that exhibit oral epithelial dysplasia (Scully 2009). A visual screen is not surgically invasive, is painless and has been found to be socially acceptable. Additional Table 1 highlights the different types of PMDs that were considered by the WHO's Working Party on Oral Cancer and Precancer to be important (Warnakulasuriya 2007). The most common form of PMD is leukoplakia (Napier 2008), which has an estimated global prevalence of 2.6% (95% confidence interval (CI) 1.72% to 2.74%) (Petti 2003). However, the extent and rate of progression of dysplasia in leukoplakia is not uniform and can vary from site to site and within the same lesion (Napier 2008).
1. Important potentially malignant disorders (PMDs).
The following were identified as PMDs by the World Health Organization's Working Group on Oral Cancer (Warnakulasuriya 2007).
|
The overall malignant transformation rate for oral leukoplakia is up to 5% (Scully 2009; van der Waal 2009), but the lack of uniformity in the extent and the rate of dysplastic change in PMDs means that predicting malignant transformation is problematic. However, there remains a consensus in the literature that the majority of cancers are preceded by a detectable pre‐clinical phase (Napier 2008).
Although there have been no randomised controlled trials (RCTs) in any developed or low‐prevalence populations (Brocklehurst 2010a), Speight et al demonstrated using a simulated model that an oral examination of high‐risk individuals may be a cost‐effective screening strategy (Speight 2006). A recent diagnostic test accuracy review looking at the accuracy of conventional oral examination as a screening test in primary settings found sensitivity estimates to range from 0.50 (95% CI 0.07 to 0.93) to 0.99 (95% CI 0.97 to 1.00) with specificity estimates 0.98 (95% CI 0.92 to 1.00) to 0.99 (95% CI 0.99 to 0.99) (Walsh 2013). Positive predictive values ranged from 0.31 to 0.86; negative predictive values ranged from 0.96 to 0.99 (Walsh 2013). Other adjunctive and diagnostic aids can be grouped into visual staining (toluidine blue), oral cytology using brush biopsy and a number of light‐based techniques (e.g. ViziLite (Zila Pharmaceuticals, AZ, USA) and VELscope (LED Dental Inc, BC, Canada)) (Brocklehurst 2010a).
Why it is important to do this review
The Cochrane Oral Health Group undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important ones to maintain on the Cochrane Library (Worthington 2015). Consequently, this review was identified as a priority title by both the oral medicine and public health expert panels (Cochrane OHG priority review portfolio).
The RCT provides the strongest level of evidence on which to base clinical decisions (Clarkson 2003) and so represents a level of rigor that is appropriate for assessing the effectiveness of any intervention or programme. As with other cancers, screening for oral cancer and PMDs has potential advantages and disadvantages (Speight 1992). Screening and treatment may offer the opportunity to reduce the incidence of invasive lesions and also could help in decreasing the mortality rates associated with oral cancer. Speight et al demonstrated that targeting high risk groups could result in a pronounced increase in the Quality Adjusted Life Years saved and any associated stage shifts could produce significant cost savings (Speight 2006). However, screening also has the potential to generate false positives and false negatives (Wilson 1968). Earlier versions of this Cochrane review concluded that there was insufficient evidence to support or refute the use of screening for oral cancer in the general population (Kujan 2003; Kujan 2006; Brocklehurst 2010c). The purpose of this latest update was to determine whether the evidence base had changed.
Objectives
To assess the effectiveness of current screening methods in decreasing oral cancer mortality.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of screening programmes for the early detection of oral cancer or potentially malignant disorder (PMD), which report on the associated mortality rates subsequent to the screen.
Types of participants
Participants involved in population, selective (high‐risk) or opportunistic screening programmes were included.
Types of interventions
Any health technology used in a screening programme for the detection of oral cancer or PMD:
visual screening;
visual staining using toluidine blue;
oral cytology using brush biopsies;
fluorescence imaging and light‐based techniques.
As this was not a diagnostic test accuracy review, the definition of a positive case in each of these categories was not defined; each study was assessed on an individual study‐by‐study basis.
Types of outcome measures
The primary outcome measure for this review was oral cancer mortality.
Other outcomes included were:
incidence of oral cancer or PMD;
stage at diagnosis;
adverse effects (outcomes from false positive or false negative results, if known);
cost data (where reported).
Search methods for identification of studies
Electronic searches
For the identification of studies included or considered for this review, detailed search strategies were developed for each database searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database (Appendix 1). The search strategies for MEDLINE and CANCERLIT used a combination of controlled vocabulary and free text terms. They were linked with the Cochrane Highly Sensitive Search Strategies (CHSSS) for identifying RCTs in MEDLINE: sensitivity maximising versions (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in boxes 6.4.a and 6.4.c of the Cochrane Handbook forSystematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011). The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs.
Databases searched
We searched the following electronic databases.
The Cochrane Oral Health Group's Trials Register (to 22 July 2013) (Appendix 2).
CENTRAL (The Cochrane Library 2013, Issue 6) (Appendix 3).
MEDLINE via OVID (1946 to 22 July 2013) (Appendix 1).
EMBASE via OVID (1980 to 22 July 2013) (Appendix 4).
CANCERLIT via PubMed (1950 to 22 July 2013) (Appendix 5).
Searching other resources
The following journals were handsearched for this review to 2010:
Oral Oncology
British Dental Journal
Cancer
Cancer Research
Community Dental Health
Community Dentistry and Oral Epidemiology.
For this update, only handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL was included. See the Cochrane Masterlist of handsearched journals for information on journals and issues searched to date.
Language
There were no non‐English papers that required translation. Had such trials been identified they would have been translated through The Cochrane Collaboration.
Unpublished trials
The bibliographies of included papers and relevant review articles were checked for studies not identified by the search strategies above. The authors of identified and included studies were also contacted to identify unpublished or ongoing trials.
Data collection and analysis
Selection of studies
The titles and abstracts obtained from initial electronic searches were scanned for relevance independently by two of the review authors (Paul Brocklehurst (PRB), Anne‐Marie Glenny (AMG)). Reports from the studies that fulfilled the inclusion criteria were obtained. When there was insufficient data in the study title to determine whether a study fulfilled the inclusion criteria, the full report was obtained and assessed independently by the same review authors. Disagreement was resolved by discussion.
Data extraction and management
All studies meeting the inclusion criteria underwent data extraction and an assessment of risk of bias was made. Studies rejected at this and subsequent stages were recorded in the table of excluded studies. Data from each included study was extracted independently using the tool developed and reported in Kujan 2005. Differences were again resolved by discussion. If a single publication reported two or more separate studies, then each study was extracted separately. If the findings of a single study were spread across two or more publications, then the publications were extracted as one. For each study with more than one control or comparison group for the intervention, the results were extracted for each intervention arm. For each trial the following data were recorded.
Year of publication, country of origin and source of study funding.
Details of the participants including demographic characteristics and criteria for inclusion.
Details on the type of intervention and comparisons.
Details on the study design.
Details on the outcomes reported, including method of assessment.
Assessment of risk of bias in included studies
Assessment of risk of bias was conducted by three review authors (PRB, AMG, Lucy O'Malley (LO)) using the Cochrane risk of bias assessment tool. The domains that were assessed for each included study were: sequence generation, allocation concealment, blinding, completeness of outcome data, risk of selective outcome reporting and risk of other potential sources of bias.
A description of the domains was tabulated for each included trial, along with a judgement of the risk of bias in accordance with the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). A summary assessment of the risk of bias across all the domains for each study was then undertaken (Higgins 2011).
Low risk of bias ‐ a low risk of bias for all key domains.
Unclear risk of bias ‐ an unclear risk of bias for one or more key domains.
High risk of bias ‐ a high risk of bias for one or more key domains.
Measures of treatment effect
For dichotomous outcomes, the estimate of effect was expressed as risk ratios with 95% confidence intervals; for continuous outcomes, mean differences were used with 95% confidence intervals.
Unit of analysis issues
The analysis for cluster randomised trials was undertaken, when feasible, at the same level of randomisation, or at an individual level with the effect of clustering being accounted for.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was assessed by means of Cochran's test for heterogeneity, considered statistically significant at the level of P value < 0.1 (Higgins 2011). The percentage total variation across the included studies was used to quantify heterogeneity and expressed as I2, with a value over 50% representing substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
Publication bias would have also been assessed using funnel plot asymmetry (Higgins 2011).
Data synthesis
Meta‐analyses would have been undertaken if the number of trials had exceeded a minimum of three. Risk ratios would have been combined for dichotomous data, and mean differences for continuous data using a random‐effects model, if data had allowed.
Results
Description of studies
A total of 3239 citations were identified through the MEDLINE searches. The full text of 30 articles were retrieved. Following further screening, 26 of these were excluded with reasons (Characteristics of excluded studies). One of the excluded studies did undertake a community‐based randomised controlled trial to examine the efficacy of toluidine blue in Taiwan (Su 2010). However, the primary aim was to determine whether toluidine blue enhanced the detection rate for potentially malignant disorders (PMD), not mortality rate. Although they did link the examined cohort with the National Cancer Registry to determine five‐year follow‐up, the power calculation was based on the former not the latter. As a result, the study was excluded from this review but included in an accompanying diagnostic test accuracy review (Walsh 2013). Only one study (four reports) met the inclusion criteria (Sankaranarayanan 2000). The principal investigator of the included trial (Dr Sankaranarayanan) was also contacted and no other relevant trials were identified (Figure 1).
1.
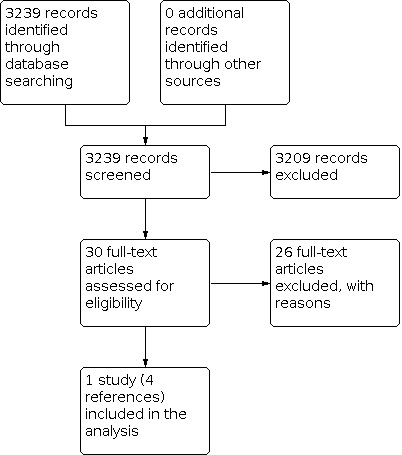
Study flow diagram.
The included study (Trivandrum Oral Cancer Screening Study; Sankaranarayanan 2000) was designed to have an 80% power at the 5% significance level to detect a 35% reduction in the cumulative mortality rate of oral cancer in 12 years of enrolment between the intervention and the control groups. The study commenced in October 1995 and three rounds of screening at three‐year intervals were planned for the study. The first round was completed in May 1998 and the second was completed in June 2002. The third round was completed in October 2004. A final round of screening was completed in 2009.
All participants (n = 191,873) were apparently healthy residents aged 35 years or older and lived in 13 rural clusters around Trivandrum City, Kerala, India, The mean number of eligible participants in each cluster was 14,759. These clusters were allocated into an intervention arm (n = 7) and a control arm (n = 6) by a blocked randomisation process. Those residents who were bedridden, suffering from open tuberculosis or other debilitating diseases were excluded alongside those participants who had already been diagnosed with oral cancer prior to entry into the study.
In the intervention arm, non‐medical university graduates were initially trained and were provided with two simple manuals on oral visual examination with colour photographs and descriptions of various oral lesions. Eligible participants were interviewed and information relating to demographic, social and personal habits including the use of paan, tobacco, alcohol and dietary supplements was recorded. Tobacco and alcohol cessation advice was provided as appropriate. Oral visual inspections were performed in daylight with the help of a flashlight. All the intra‐oral sites were carefully examined and palpated and the neck was also palpated to detect enlarged lymph nodes. The findings were recorded as normal, non‐referable lesions and referable lesions.
Participants who had a positive screen were referred for examination by a dentist or physician for confirmation. It is unclear whether these clinicians were also trained in the recognition of oral cancer or PMDs. Oral biopsies were performed in those with clinically confirmed homogeneous leukoplakias, non‐homogeneous leukoplakias, oral submucous fibrosis and oral cancers. Surgical excision was undertaken for leukoplakia wherever possible. All PMDs were reviewed regularly.
In the control arm, participants were visited by a "control health worker" who recorded the same sociodemographic information and measured height, weight, blood pressure and respiratory peak flow measurements. The health workers in the control arm were not trained to undertake a visual oral inspection.
Oral cancer mortality was reported as the main outcome measure.
Of the 96,517 eligible subjects in the intervention arm, 25,144 (26.1%) had one, 22,382 (23.2%) had two, 22,008 (22.8%) had three and 19,288 (20.0%) had four cycles of screening. 49,179 (51.0%) individuals were screened in the first round and 55,993 (58.0%), 64,898 (67.2%) and 43,014 (44.6%) were screened in the second, third and fourth cycles respectively. The participation rate (at least one screen) for this group was 88,822 (92%); males (86%) and females (94%). Of the 95,356 eligible subjects in the control group, 43,992 (46.1%) were screened in the fourth cycle.
Demographic details were not provided for the final (fourth) cycle of screening, but were provided for the first three cycles (Additional Table 2). Across the period of the study (all four cycles), 6.3% (n = 5586) of subjects screened as part of the intervention group had a referable lesion and 59% (n = 3298) of these screen positive subjects complied with referral (Additional Table 3). The control group consisted of 95,356 persons. 46% (n = 43,992) of the control group were screened in the final (fourth) cycle and 2.6% (n = 1163) of these were found to have a referable lesion with 16.3% (n = 189) complying with referral.
2. Comparison of risk factors between the intervention and control groups after three cycles (nine years) follow‐up.
| Trivandrum Oral Cancer Screening Study | Screening group | Control group |
| Number of interviewed participants | n = 7 clusters (87,829: 91%) | n = 6 clusters (80,086: 84%) |
| Gender | 35,687 male 52,142 female |
31,281 male 48,805 female |
| Income (< 1500 rupees (USD 35) per month) | 42,415 (49%) | 30,849 (40%) |
| Occupation (manual workers) | 68,645 (78%) | 55,811 (71%) |
| Education | 68,263 (78%) | 64,291 (78%) |
| Age (years; mean (SD, range)) | 49 (0.7, 48‐50) | 49 (0.8, 48‐50) |
| No habits | 10,933 male (27%) 39,923 female (73%) |
13,996 male (33%) 42,361 female (79%) |
| Chewing habits | 12,329 male (30%) 14,570 female (27%) |
10,586 male (24.9%) 10,748 female (20%) |
| Smoking habits | 26,133 male (63%) 1610 female (3%) |
23,270 male (56%) 609 female (1%) |
| Drinking habits | 17,738 male (43%) 133 female (0.2%) |
15,472 male (37%) 127 female (0.1%) |
SD = standard deviation. Note: Data on risk factors not available after 4 cycles (15 years), but authors report that the participants had a similar distribution.
3. Screening history after four cycles (15 years) follow‐up.
| Screening history | Screening group | Control |
| Number recruited | 7 clusters (n = 96,517) |
6 clusters (n = 95,356) |
| Not screened | 7695 | 51,365 |
| Screened once | 25,144 | 43,992 |
| Screened twice | 22,382 | |
| Screened three times | 22,008 | |
| Screened four times | 19,288 | |
| Number of screen positive individuals | 5586 | 1163 |
| Individuals complied with referral | 3298 | 189 |
Risk of bias in included studies
Allocation
Sequence generation
The randomisation procedure was conducted using restricted block randomisation. The exact detail of this process was not provided, although the clusters were grouped into blocks of four and allocated at random to screening or non‐screening groups from the six possible combinations available to each block of four. Clustering was not accounted for in the analysis.
Allocation concealment
No detail of allocation concealment was provided, although the principal investigator confirmed that this was not undertaken.
Blinding
Blinding of outcome assessment
No blinding was undertaken in the study, but the review authors judge that the outcome and its measurement are unlikely to be influenced by this. As a result, the risk of bias based on the lack of blinding is considered to be low.
Incomplete outcome data
Withdrawals and drop‐outs were not described clearly and missing data will have increased the risk of bias. Of those who were referred with positive lesions, 59% of individuals in the screening group complied with referral and 16% in the control group (Additional Table 3). The analysis was carried out on an intention‐to‐treat (ITT) basis.
Selective reporting
The study protocol was not made available, but it appears that the published reports include all the expected and pre‐specified outcome measures.
Other potential sources of bias
Positive cases were referred to dentists and physicians to make a diagnosis, but it is unclear whether standardised criteria were used by these clinicians or whether they had received any formal training. It is stated that subjects with confirmed oral cancer and PMDs were biopsied and those with confirmed oral cancer were referred. However, this detail was absent and only 26.4% and 26.0% of subjects with a PMD had a biopsy in the second or third cycle respectively. It is not clear whether all suspected oral cancer cases did receive a biopsy, but given the definition of "interval cases" in the third paper, it would appear not.
In addition, it is stated in the third paper that the reference investigation for final diagnosis was clinical examination by physicians or histology or both. As it is not possible to diagnose early malignancy by visual appearance alone, this may have led to substantial under‐reporting of oral cancer. The lack of a histological diagnosis for many of the PMDs also makes it difficult to accurately assess the correct diagnosis and true prevalence of these disorders. Prevalence of PMDs is provided in detail for the first two cycles only. The fourth paper presents incidence and mortality data for each round of screening. Neither the third or fourth papers present data regarding prevalence of PMDs.
In the included study the health workers reported on 24 baseline variables including multiple age strata, occupation, education, income, household belongings such as television and personal habits of chewing, smoking, and drinking. The intervention and control cohorts appear to have been well matched for the stratified variable age at the baseline. However, the distribution of income, education, use of tobacco and alcohol varied across the intervention and control groups, with the former demonstrating higher levels of consumption (Additional Table 2). Men smoked and drank alcohol more than females in both groups, but the prevalence of chewing tobacco was not as marked across gender differences. Although, such differences in baseline variables might be expected to occur in cluster randomised studies, the differences between the numbers who used tobacco and alcohol need to be borne in mind when interpreting the results.
Effects of interventions
The included study reported data on oral cancer incidence, disease‐specific mortality, and stage at diagnosis after 15‐years follow‐up. Data on quality of life and all cause mortality were not reported.
Oral cancer mortality
There was a 12% reduction in oral cancer mortality between the intervention and control arms, but this difference was not statistically significant. Over the four cycles (15 years), 138 of 279 subjects with oral cancer in the intervention group and 154 of the 244 cases in the control group died, which represents a mortality rate of 15.4 and 17.1 per 100,000 person‐years respectively (risk ratio (RR) 0.88; 95% confidence interval (CI) 0.69 to 1.12). Age‐adjusted rates were 18.9 per 100,000 person‐years in the intervention group and 19.7 per 100,000 person‐years in the control (Additional Table 4).
4. Oral cancer experience after four cycles (15 years) follow‐up.
| Trivandrum Oral Cancer Screening Study | Screening group | Control group | Risk ratio (95% confidence interval) |
| Total number participants | 96,517 | 95,356 | |
| Person‐years of observation | 895,310 | 898,280 | |
| Number of oral cancers | 279 | 244 | |
| Screen detected cases | 188 | Nil | |
| Deaths from oral cancer | 138 | 154 | |
| Case fatality rate | 49.5% | 63.1% | |
| Crude incidence rate / 100,000 person‐years of observation | 31.2 | 27.2 | 1.14 (0.91 to 1.44) |
| Age‐standardised incidence rate / 100,000 person‐years | 37.1 | 30.8 | |
| Crude mortality rate from oral cancer /100,000 person‐years of observation | 15.4 | 17.1 | 0.88 (0.69 to 1.12) |
| Age‐standardised mortality rate / 100,000 person‐years | 18.9 | 19.7 | |
| Proportion of cancers at stage III or worse1 | 52.6% | 65.2% | 0.81 (0.70 to 0.93) |
1Does not include individuals for whom stage unknown (22 in screening group; 19 in control group).
There was a 24% reduction in oral cancer mortality between the intervention and control arms for those participants who used tobacco or alcohol or both and this difference was statistically significant. Over the four cycles (15 years), 129 of 254 subjects with oral cancer in the intervention group and 147 of the 232 cases in the control group died, which represents a mortality rate of 30.0 and 39.0 per 100,000 person‐years respectively (RR 0.76; 95% CI 0.60 to 0.97) (Additional Table 5). Age‐adjusted rates were 29.1 per 100,000 person‐years in the intervention group and 37.1 per 100,000 person‐years in the control (Additional Table 4).
5. Oral cancer experience in high‐risk individuals after four cycles (15 years) follow‐up.
| Trivandrum Oral Cancer Screening Study | Screening group | Control group | Risk ratio (95% confidence interval) |
| Person‐years of observation | 429,620 | 377,350 | |
| Number of oral cancer cases | 254 | 232 | |
| Deaths from oral cancer | 129 | 147 | |
| Case fatality rate | 50.8% | 63.4% | |
| Crude incidence rate/ 100,000 person‐years | 59.2 | 61.6 | 0.97 (0.79 to 1.19) |
| Age‐standardised incidence rate/ 100,000 person‐years | 57.3 | 58.5 | |
| Crude mortality rate/ 100,000 person‐years | 30.0 | 39.0 | 0.76 (0.60 to 0.97) |
| Age‐standardised mortality rate/ 100,000 person‐years | 29.1 | 37.1 | |
| Age‐standardised mortality rate/ 100,000 person‐years | 18.9 | 19.7 | |
| Proportion of cancers at stage III or worse1 | 54.3% | 66.4% | 0.82 (0.71 to 0.95) |
1Does not include individuals for whom stage unknown (22 in screening group; 19 in control group).
Although data presented after four cycles (15 years) were not divided by gender, the three‐year cycle data (nine years) showed a significant reduction of 43% in mortality rates for men from 42.9 per 100,000 person‐years in the control group to 24.6 per 100,000 person‐years in the intervention group. For women, there was a 22% reduction, from 50.7 to 39.4 per 100,000 person‐years, but this did not reach significance.
For the participants that adhered to all four cycles of the screening programme, there was a 79% reduction in oral cancer mortality (81% amongst the users of tobacco or alcohol or both) compared to the control, which was statistically significant (Additional Table 6).
6. Oral cancer mortality rate by number of times screened.
| Risk type | Arm | Cycles | Mortality rate* | Hazard ratio (95% confidence interval)** |
| All participants | Control | n/a | 17.1 | 1.00 |
| Screening group | 0 | 37.2 | 1.46 (0.78 to 2.73) | |
| 1 | 44.1 | 2.26 (1.66 to 3.09) | ||
| 2 | 16.2 | 0.94 (0.68 to 1.30) | ||
| 3 | 10.4 | 0.62 (0.37 to 1.04) | ||
| 4 | 3.0 | 0.21 (0.13 to 0.35) | ||
| High‐risk participants | Control | n/a | 39.0 | 1.00 |
| Screening group | 0 | 59.4 | 1.27 (0.68 to 2.37) | |
| 1 | 74.7 | 1.90 (1.45 to 2.49) | ||
| 2 | 31.0 | 0.83 (0.62 to 1.12) | ||
| 3 | 20.6 | 0.53 (0.34 to 0.84) | ||
| 4 | 7.1 | 0.19 (0.11 to 0.31) |
*Per 100,000 person‐years of observation
**Adjusted for age, sex and number of residents.
Oral cancer incidence
Among the 96,517 participants screened in the intervention group, 5586 (6.3%) were found to have referable lesions. Of these, 3298 (59%) complied with the referral criteria for confirmatory examination by dentists or medical officers in special clinics. Healthy mucosa or benign lesions were found in 770 (23.3%). The number of PMDs was 2336 (70.8%) (lichen planus (n = 53), homogenous leukoplakia (n = 898) and submucous fibrosis (n = 573)) and growths suspicious of oral cancer 192/3298 (4%). Of those diagnosed with PMDs, 21.4% (n = 499), underwent biopsies and 4.4% (n = 22) were confirmed squamous cell carcinoma. Of those diagnosed with suspicious growths, 84.9% (n = 163) were confirmed as squamous cell carcinoma and 1.6% (n = 3) as verrucous carcinoma.
The detection rate of PMD and oral cancer in the first, second, and third and fourth rounds of screening were 28.0, 11.9, 11.6 and 3.9 per 1000 screened subjects respectively. The crude incident rate of oral cancer was 31.2 per 100,000 person‐years in the screening group and 27.2 per 100,000 person‐years in the control group, with a risk ratio of 1.14 (95% CI 0.91 to 1.44). Age‐adjusted incidence rates were 37.1 per 100,000 person‐years and 30.8 per 100,000 person‐years respectively (Additional Table 4).
Test performance
Across the four cycles (15 years) of the programme, the reported sensitivity of the visual examination in detecting oral cancer was 67.4% (188/279) (Additional Table 4). No information on the specificity or the positive predictive value of the programme was recorded.
Survival
Survival rates were calculated by comparing the proportion of patients alive at five years after diagnosis across the two groups. A significantly higher five‐year survival rate was reported in the screened group (55.5%) compared to the control (43.4%) (P value = 0.003).
Stage shift at diagnosis
There was a statistically significant stage shift in the cancers that were diagnosed in the screened group, based on the criteria of the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC). In the screened group, 147/279 (52.6%) cases were in stage III or worse at diagnosis, as opposed to 159/244 (65.2%) of cases in the control group (RR 0.81; 95% CI 0.70 to 0.93) (Additional Table 4; Additional Table 7). For users of tobacco, alcohol or both, 138/254 (54.3%) cases were in stage III or worse at diagnosis in the screened group, as opposed to 154/232 (66.4%) of cases in the control group (RR 0.82; 95% CI 0.71 to 0.95) (Additional Table 5; Additional Table 7).
7. Distribution of stage over four cycles (15 years).
| Arm | Cycles | Clinical stage | Total | ||||
| I | II | III | IV | Unknown | |||
| Intervention group | Baseline | 0 | 3 (16%) | 3 (16%) | 7 (37%) | 6 (32%) | 19 |
| 1 | 9 (11%) | 10 (13%) | 18 (23%) | 32 (41%) | 10 (13%) | 79 | |
| 2 | 10 (15%) | 11 (17%) | 17 (26%) | 25 (38%) | 3 (5%) | 66 | |
| 3 | 23 (32%) | 12 (17%) | 9 (13%) | 25 (35%) | 3 (4%) | 72 | |
| 4 | 17 (40%) | 15 (35%) | 4 (9%) | 7 (34%) | 0 | 43 | |
| Total | 59 (21%) | 51 (18%) | 51 (18%) | 96 (34%) | 22 (8%) | 279 | |
| Control group | 1996 ‐ 2004 (detected not due to screening) | 21 (13%) | 19 (12%) | 34 (22%) | 72 (46%) | 12 (8%) | 158 |
| Not screened | 0 | 7 (16%) | 12 (27%) | 20 (44%) | 6 (13%) | 45 | |
| Screened | 10 (24%) | 9 (22%) | 6 (15%) | 15 (37%) | 1 (2%) | 41 | |
| Total | 31 (13%) | 35 (14%) | 52 (21%) | 107 (44%) | 19 (8%) | 244 | |
Cost‐effectiveness
The costs associated with the screening programme were reported after three cycles (nine years) (Subramanian 2009) (Additional Table 8). The benefit produced by a screen was 269.31 life‐years saved per 100,000 for all the individuals and 1437.64 for those at high risk. The incremental cost per life‐year saved was USD 835 for all individuals, which reduced to USD 156 for high‐risk individuals. This fulfils the target set by the World Health Organization (WHO) Commission on Macroeconomics and Health (WHO 2001), who define an intervention to be cost‐effective when its cost‐effectiveness ratio is less than a country's gross domestic product per capita. Subramanian argues that this provides good evidence that opportunistic screening of high‐risk groups is cost‐effective (Subramanian 2009).
8. Cost‐effectiveness of the screening programme after three cycles (nine years).
| Detail | Cost of intervention less cost of control (US$) | |
| Total cost per 100,000 individuals | 224,964 | |
| Cost per additional cancer detected by the screen | All individuals | 4817 |
| Cost per additional cancer detected by the screen | High‐risk individuals | 9394 |
| Cost per life‐year saved by the screen | All individuals | 835 |
| Cost per life‐year saved by the screen | High‐risk individuals | 156 |
Costs based on the calender year of 2004 (Subramanian 2009).
Discussion
The incidence of oral cancer is increasing in low, middle and high‐income countries (Warnakulasuriya 2009). Delays in diagnosis and management persist (Onizawa 2003McLeod 2005) and are associated with a dramatic deterioration in five‐year survival rates. Effective primary and secondary prevention strategies are critical in delivering the World Health Organization's (WHO) resolution that oral cancer should be an integral part of national cancer control programmes (Petersen 2009).
Given that the majority of oral carcinomas are preceded by visible lesions (Scully 2009), determining the efficacy and effectiveness of screening warrants attention, whilst balancing the potential benefits with any potential negative consequences of any programme (Wilson 1968).
Summary of main results
The study reported a sensitivity of the visual examination in detecting oral cancer was 67.4%. However, the data for users of tobacco, alcohol or both demonstrated a reduction in mortality rates of 24% after four cycles (15 years), which was statistically significant. In addition, there was a statistically significant difference in the number of stage III cancers between the intervention and control arms, suggesting that the screening programme was identifying cancers at an early stage. When these results are combined with the significant stage shift and survival rate in the intervention group, it would appear that visual examination could be effective at reducing mortality rates for oral cancer when used within a targeted screening programme. However, the included study had a high risk of bias.
Overall completeness and applicability of evidence
The purpose of health care is to improve both the quantity and quality of life (Kaplan 2005). The evidence from the Kerala trial (Sankaranarayanan 2000) is that visual screening can reduce the mortality rate in users of tobacco, alcohol or both and can produce a stage shift. Given that late stage disease is recognised as a major contributory factor for cancer survival (Onizawa 2003; McLeod 2005), it would appear that the screening of high‐risk individuals could be warranted. However, the evidence from this study stems from a population with a high incidence of oral cavity cancer, and its applicability to other countries with lower incidence rates is unknown. In addition, the efficacy of the early management of potentially malignant disorders (PMDs) is a controversial area (Holmstrup 2007; Holmstrup 2009). Holmstrup argues that even if early lesions are surgically removed, the risk of malignant change can remain as a result of "field change" i.e. the lesion represents only a small area of a wider field of damaged mucosa (Holmstrup 2007; Holmstrup 2009).
The trial used non‐medical university graduates, trained specifically to perform visual inspection of the oral mucosa, with the help of a flashlight. The screening was undertaken in individuals own home, with the health workers going 'door‐to‐door'. The ability to translate this screening model to other settings is unclear. However, the Kerala study does demonstrate the potential of allied health professionals to screen for oral cancer and PMDs. This is becoming increasingly relevant as more regulators allow patients to directly access allied providers of dental care (oral health practitioners), in addition to the dentist.
The cost‐effectiveness of the Kerala study was reported after three cycles (nine years) (Subramanian 2009), demonstrating that 1437.64 life‐years could be saved per 100,000 high‐risk individuals, with an incremental cost per life‐year saved of USD 156. According to the authors, this fulfils the target set by the WHO Commission on Macroeconomics and Health, who define an intervention as being cost‐effective when its cost‐effectiveness ratio is less than a country's gross domestic product per capita. However, these results also need to be read in context of the relative prevalence of the condition and account for the potential problem of compliance; out of the 5586 participants that were screened positive, only 59% (n = 3298) complied with referral.
The consideration of both the benefits and harms of screening is an essential component of any programme (Wilson 1968) and is fundamental to the satisfactory introduction of any technology into daily practice (Duffy 2001). The sensitivity reported in the Kerala study was relatively low (67%). In addition, false positives can have unintended psychological consequences, for example, increasing anxiety and exposing the patient to unnecessary further investigations. However, these could be reduced by careful patient management and by educating screened patients about the positive benefits of screening (Speight 1992). Recent studies by Brocklehurst, highlighted the need to train general dental practitioners to discuss positive findings (Brocklehurst 2010b) and the need for standardised criteria to avoid both under and over‐referral in clinical practice (Brocklehurst 2010).
Quality of the evidence
The Kerala study had a number of methodological weaknesses that may have introduced bias. These included a lack of detail about the process of sequence generation to ensure random assignment, no analysis of the impact of clustering on the results and no detail about allocation concealment. In addition, there was no blinding of the outcome assessment and withdrawals and drop‐outs were not described. More importantly, only 59% of individuals with screen‐positive lesions in the intervention arm complied with referral and it was unclear whether the clinicians who saw these patients followed any standardised criteria. In addition, only 26.4%, 26.0%, and 21.4% of subjects had a biopsy in the second, third cycle or fourth cycle respectively, with no detail being provided from the first cycle. As it is not possible to diagnose early malignancy by visual appearance alone, this may have led to substantial under‐reporting of oral cancer and the lack of a histological diagnosis makes it difficult to accurately assess the correct diagnosis and true prevalence of these disorders. It has been argued that the small number of randomised clusters could also produce statistical heterogeneity and the close geographical proximity of the clusters may have led to contamination (Kujan 2005).
Potential biases in the review process
We conducted a broad search of several databases and placed no restrictions on the language of publication when searching the electronic databases or reviewing reference lists of included studies. All data extraction and risk of bias assessment was conducted in duplicate.
Agreements and disagreements with other studies or reviews
A meta‐analysis of visual screening found a weighted and pooled sensitivity of 84.8% (95% confidence interval (CI) 73.0 to 91.9) and specificity of 96.5% (95% CI 93.0 to 98.2) (Downer 2004). However, there was considerable heterogeneity in the studies pooled due to differences in the size of the target populations and it is arguable that a meta‐analysis was inappropriate. A more recent review has evaluated the diagnostic accuracy of non‐specialist conventional oral examination, vital rinsing, light‐based detection, biomarkers and mouth self examination for the identification of individuals with suspected oral squamous cell carcinoma or PMD. Sensitivity values for studies for all tests were varied and relatively imprecise with sensitivity values ranging from 0.59 (0.39 to 0.78) to 0.99 (95% CI 0.97 to 1.00) for the conventional oral examination (Walsh 2013).
These values of sensitivity and specificity for visual examination have not been surpassed by any other type of method, such as self examination, vital staining (toluidine blue), oral cytology or light‐based techniques (Lingen 2008; Patton 2008; Walsh 2013). The review by Walsh et al showed sensitivity values ranging from 0.18 (95% CI 0.13 to 0.24) to 0.33 (95% CI 0.10 to 0.65) for mouth self examination and a sensitivity value of 0.20 (95% 0.01 to 0.72) for the addition of toluidine blue (Walsh 2013). In Patton's systematic review, 23 studies met the inclusion criteria, yet there remained insufficient evidence to support or refute the use of adjunctive techniques to the visual examination (Patton 2008). In another review, Lingen found that the majority of the published studies had employed these techniques on patients who had already received a diagnosis and that they did not improve upon the sensitivity or specificity of the visual examination (Lingen 2008).
Authors' conclusions
Implications for practice.
The results suggest that there is insufficient evidence to recommend a whole population screening programme for oral cancer. However, the results from the Kerala study suggest that a targeted population approach could reduce the mortality rate and produce a stage shift, but the risk of bias in the included study means that further well‐designed randomised controlled trials are necessary to establish the validity of this relationship.
In the meantime, opportunistic visual screening by appropriately trained dentists and oral health practitioners is recommended for all patients and particularly for those who use tobacco, alcohol or both. Systematic examination of the oral cavity by front‐line health workers should remain an integral part of their routine for routine recall appointments.
Implications for research.
Given the high risk of bias in the study included in this review, a lack of randomised controlled studies associated with adjunctive methods (e.g. brush biopsy, fluorescence imaging) and a lack of understanding of the natural history of oral cancer, further randomised controlled trials are recommended. These should ensure the method of randomisation is accounted for in the analysis, that there is adequate allocation concealment, a standardised intervention and a clear follow‐up procedure.
What's new
| Date | Event | Description |
|---|---|---|
| 17 March 2021 | Review declared as stable | There is no new evidence for this review and it will no longer be updated. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 12 September 2013 | New citation required but conclusions have not changed | This version includes a change in authors. Review text, methodology, background and references brought up to date. |
| 12 September 2013 | New search has been performed | Searches updated on 22 July 2013. |
| 6 October 2010 | New search has been performed | New searches and methodology. Review text, background and references brought up to date. |
| 6 October 2010 | New citation required but conclusions have not changed | New authorship. |
| 25 May 2006 | New citation required but conclusions have not changed | This version includes a change in authors. |
| 23 May 2006 | New search has been performed | The current review reflects the results of an update search conducted in July 2005. No new trials were identified as meeting the review's inclusion criteria. However, a trial presenting the final analysis for the one, previously included trial was identified. The conclusions of the review remain the same. |
Notes
As of March 2021, there is no new evidence to include in this review and it will no longer be updated.
Acknowledgements
We would like to thank Dr Sankaranarayanan for answering the queries of the review group. We would also like to thank Anne Littlewood (Cochrane Oral Health Group) for her help with preparing the searches for this update. We are also grateful to Professor Helen Worthington for her assistance on the development of the initial review and the Editors at the Cochrane Oral Health Group for their valuable comments.
Appendices
Appendix 1. MEDLINE via OVID search strategy
1. exp MOUTH/ 2. exp LIP/ 3. exp GINGIVA/ 4. exp TONGUE/ 5. exp OROPHARYNX/ 6. exp HYPOPHARYNX/ 7. exp PALATE/ 8. exp CHEEK/ 9. (mouth or lip$ or tongue$ or gingiv$ or oropharynx or palate or cheek$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 10. or/1‐9 11. exp MOUTH NEOPLASMS/ 12. exp PRECANCEROUS CONDITIONS/ 13. (tumor$ or tumour$ or cancer$ or carcinoma$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 14. malignan$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 15. dysplasia$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 16. (oral adj6 cancer$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 17. or/11‐16 18. MASS SCREENING/ 19. (visual$ adj screen$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 20. tolonium chloride.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 21. TOLONIUM CHLORIDE/ 22. "toluidine blue".mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 23. exp TOLUIDINES/ 24. "toluidine dye".mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 25. ("brush biopsy" or "exfoliate cytology").mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 26. "fluorescent imaging".mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 27. ("fluorescent dye$" or "fluorescent antibody technique" or fluorescence).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 28. prevent$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 29. screen$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 30. (early adj3 detect$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 31. or/18‐30 32. 10 and 17 and 31
The above subject search was linked to the the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health Group's Trials Register search strategy
From 2013, searches of the Cochrane Oral Health Group's Trials Register were updated using the Cochrane Register of Studies software and the search strategy below:
#1 (tumor* or tumour* or cancer* or carcinoma* or malignan*):ti,ab #2 (screen* or tolonium or "brush biopsy" or "exfoliative cytology" or fluorescen* or "early detect*"):ti,ab #3 #1 and #2
Previous searches of the Oral Health Group's Trials Register were undertaken using the Procite software and the search strategy below:
((tumor* or tumour* or cancer* or carcinoma* or malignan*) AND (screen* or tolonium or "brush biopsy" or "exfoliative cytology" or fluorescen* or "early detect*"))
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor MOUTH explode all trees #2 MeSH descriptor LIP explode all trees #3 MeSH descriptor GINGIVA this term only #4 MeSH descriptor TONGUE explode all trees #5 MeSH descriptor OROPHARYNX explode all trees #6 MeSH descriptor HYPOPHARYNX explode all trees #7 MeSH descriptor PALATE explode all trees #8 MeSH descriptor CHEEK this term only #9 (mouth* in All Text or lip* in All Text or tongue* in All Text or gingiv* in All Text or oropharnyx in All Text or palate* in All Text or cheek* in All Text) #10 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #11 MeSH descriptor MOUTH NEOPLASMS explode all trees #12 MeSH descriptor PRECANCEROUS CONDITIONS explode all trees #13 (tumor* in All Text or tumour* in All Text or cancer* in All Text or carcinoma* in All Text) #14 malignan* in All Text #15 dysplasia* in All Text #16 (oral in All Text near/6 cancer* in All Text) #17 (#11 or #12 or #13 or #14 or #15 or #16) #18 MeSH descriptor Mass Screening explode all trees #19 "visual* screen*" in All Text #20 "tolonium chloride" in All Text #21 MeSH descriptor TOLONIUM CHLORIDE this term only #22 "toluidine blue" in All Text #23 MeSH descriptor TOLUIDINES explode all trees #24 "toluidine dye" in All Text #25 ("brush biopsy" in All Text or "exfoliate cytology" in All Text) #26 "fluorescent imaging" in All Text #27 ("fluorescent dye*" in All Text or "fluorescent antibody technique" in All Text or fluorescence in All Text) #28 prevent* in All Text #29 screen* in All Text #30 (early in All Text near/3 detect* in All Text) #31 (#18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30) #32 (#10 and #17 and #31)
Appendix 4. EMBASE via OVID search strategy
1. exp MOUTH/ 2. exp LIP/ 3. exp GINGIVA/ 4. exp TONGUE/ 5. exp OROPHARYNX/ 6. exp HYPOPHARYNX/ 7. exp PALATE/ 8. exp CHEEK/ 9. (mouth or lip$ or tongue$ or gingiv$ or oropharynx or palate or cheek$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 10. or/1‐9 11. exp MOUTH NEOPLASMS/ 12. exp PRECANCEROUS CONDITIONS/ 13. (tumor$ or tumour$ or cancer$ or carcinoma$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 14. malignan$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 15. dysplasia$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 16. (oral adj6 cancer$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 17. or/11‐16 18. MASS SCREENING/ 19. (visual$ adj screen$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 20. tolonium chloride.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 21. TOLONIUM CHLORIDE/ 22. "toluidine blue".mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 23. exp TOLUIDINES/ 24. "toluidine dye".mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 25. ("brush biopsy" or "exfoliate cytology").mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 26. "fluorescent imaging".mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 27. ("fluorescent dye$" or "fluorescent antibody technique" or fluorescence).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 28. prevent$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 29. screen$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 30. (early adj3 detect$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 31. or/18‐30 32. 10 and 17 and 31
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID.
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. CANCERLIT via PubMed search strategy
#1 Search MOUTH [mh:exp] #2 Search LIP [mh:exp] #3 Search GINGIVA [mh:exp] #4 Search TONGUE [mh:exp] #5 Search OROPHARYNX [mh:exp] #6 Search HYPOPHARYNX [mh:exp] #7 Search PALATE [mh:exp] #8 Search CHEEK [mh:exp] #9 Search (mouth or lip* or tongue* or gingiv* or oropharynx or palate or cheek*) #10 Search #1 or #2 or #3 pr #4 or #5 or #6 or #7 or #8 or #9 #11 Search MOUTH NEOPLASMS [mh:exp] #12 Search PRECANCEROUS CONDITIONS [mh:exp] #13 Search (tumor* or tumour* or cancer* or carcinoma*) #14 Search malignan* #15 Search dysplasia* #16 Search "oral cancer*" #17 Search #11 or #12 or #13 or #14 or #15 or #16 #18 Search MASS SCREENING [mh:exp] #19 Search "visual* screen*" #20 Search "tolonium chloride" #21 Search TOLONIUM CHLORIDE [mh:noexp] #22 Search "toluidine blue" #23 Search TOLUIDINES [mh:exp] #24 Search "toluidine dye" #25 Search ("brush biopsy" or "exfoliate cytology") #26 Search "fluorescent imaging" #27 Search ("fluorescent dye*" or "fluorescent antibody technique" or fluorescence) #28 Search prevent* #29 Search screen* #30 Search "early detect*" #31 Search #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.a of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).
#1 randomized controlled trial [pt] #2 controlled clinical trial [pt] #3 randomized [tiab] #4 placebo [tiab] #5 drug therapy [sh] #6 randomly [tiab] #7 trial [tiab] #8 groups [tiab] #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10 animals [mh] NOT humans [mh] #11 #9 NOT #10
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Sankaranarayanan 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial, Kerala, India.
First round: 1995 to 1998.
Second round: 1998 to 2002.
Third round: 2002 to 2004. Final assessment: 2004 to 2009. |
|
| Participants | General population aged 35 years or older, all subjects 191,873 were apparently healthy residents were grouped into intervention (n = 7 clusters, 96,517) and control (n = 6 clusters, 95,356). | |
| Interventions | Health workers interviewed the eligible subjects to extract specified information. Intervention group: visual examination of the oral mucosa.
Control group: follow‐up to the end point. The intervention and control cohorts are being followed up by the Trivandrum population‐based cancer registry to determine the incidence and stage distribution of invasive oral cancer, treatment given and mortality. |
|
| Outcomes | Oral cancer mortality was the major outcome. Further outcome measures were. 1. Participation: defined as "the number of eligible subjects screened as a proportion of the total eligible in the intervention arm". 2. Positivity rate: defined as "the proportion of screened subjects identified with referable lesions". 3. Detection rate: defined as "the number of subjects with lesions detected per 1000 screened subjects in the intervention group". 4. Compliance with referral: defined as "the proportion of screen positive subjects reporting for diagnostic confirmation by dentists or physicians". 5. Sensitivity, specificity, and positive predictive values. 6. Programme sensitivity and specificity: defined as "the number of screen‐detected oral cancers as a proportion of the total oral cancers in the intervention group", "the proportion of screen true‐negative subjects among the total non‐cancer‐eligible subjects" and "the number of screen‐detected oral cancers as a proportion of total screen positive subjects" respectively. 7. Incidence rate of oral cancers. 8. Characteristics of oral cancers in the study group including: the maximum dimension of lesions, regional lymph node involvement and International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) TNM stage grouping distribution. 9. Case fatality for oral cancer cases diagnosed during the study period: defined as "the number of deaths among the total number of cases". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjects were allocated by block randomisation into 13 clusters but no detail is given about how this process was undertaken. |
| Allocation concealment (selection bias) | High risk | Author of the trial stated that there was no concealed allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the review authors judge that the outcome and its measurement are unlikely to be influenced by this. Considered low risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all participants attended for biopsy after screening (only 63% of screened positive complied with referral to have a biopsy). These missing data will have increased the risk of bias. |
| Selective reporting (reporting bias) | Low risk | Protocol is not available, but it appears that the published reports include all expected and pre‐specified outcomes. |
| Other bias | Unclear risk | Positive cases were referred to dentists and physicians to make a diagnosis, but it is unclear whether standardised criteria were used by these clinicians or whether they had received any training in identification of positive lesions. It is stated that subjects with confirmed oral cancer and PMDs were biopsied and those with confirmed oral cancer were referred. However, although not detailed in the first cycle, only 26.4% of subjects with a PMD had a biopsy in the second cycle and only 26% in the third cycle. It is not clear whether all suspected oral cancer cases did receive a biopsy, but given the definition of "interval cases" in the third paper, it would appear not. In addition, it is stated in the third paper that the reference investigation for final diagnosis was clinical examination by doctors or histology or both. As it is not possible to diagnose early malignancy by visual appearance alone, this may have led to substantial under‐reporting of oral cancer. The lack of a histological diagnosis for many of the PMDs also makes it difficult to accurately assess the correct diagnosis and true prevalence of these disorders. Prevalence of PMD and mortality data is only provided in detail for the first two cycles only. The third paper presents the results over the three cycles from 1996 to 2004 and so does not provide individual detail about the results of the third cycle. It is not clear why this was the case. |
PMDs = potentially malignant disorders.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1998 | Letter to author. |
| Chamberlain 1993 | Review study. |
| Chen 2004 | Uncontrolled clinical mass screening study. |
| Cheng 2003 | Randomised controlled trial (diagnostic use). |
| Eliezri 1988 | Uncontrolled study (secondary care). |
| Garrote 1995 | Uncontrolled study. |
| Gray 2000 | Review. |
| Gupta 1986 | Non‐randomised controlled study. |
| Gupta 1992 | Non‐randomised controlled study. |
| Ikeda 1991 | Uncontrolled study. |
| Ikeda 1995 | Uncontrolled study. |
| Lavelle 2005 | Review. |
| Martin 1998 | Uncontrolled study. |
| Miller 1988 | Study in hamsters. |
| Moyer 1986 | Uncontrolled study (diagnostic only). |
| Mullhaupt 2004 | Non‐randomised controlled study. |
| Nagao 2000 | Uncontrolled study. |
| Nagao 2000a | Uncontrolled study. |
| Nagao 2003 | Uncontrolled mass screening study. |
| Patton 2003 | Review. |
| Sankaranarayanan 1997 | Review study. |
| Sankaranarayanan 2002 | Observational, case‐control study. |
| Silverman 1984 | Uncontrolled study. |
| Su 2010 | Community‐based randomised controlled trial whose primary aim was to determine whether toluidine blue enhanced the detection rate for potentially malignant disorders. |
| Vahidy 1972 | Uncontrolled study. |
| Zhang 2005 | Observational study. |
Differences between protocol and review
None
Contributions of authors
Development of protocol based on the latest Cochrane guidance: Paul Brocklehurst (PRB). Identification of studies: PRB, Anne‐Marie Glenny (AMG), Lucy O'Malley (LO). Data extraction: PRB, AMG, LO. Assessment of risk of bias: PRB, AMG, LO. Data input/synthesis: PRB, AMG, LO. Writing of conclusions: PRB, AMG, Omar Kujan (OK), Graham Ogden (GO), Simon Shepherd (SS).
Sources of support
Internal sources
The University of Manchester, UK
AlBaath University, Syrian Arab Republic
-
Manchester Academic Health Sciences Centre (MAHSC), UK
The Cochrane Oral Health Group is supported by MAHSC and the NIHR Manchester Biomedical Research Centre
External sources
-
Cochrane Oral Health Group Global Alliance, UK
All reviews in the Cochrane Oral Health Group are supported by Global Alliance member organisations (British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; National Center for Dental Hygiene Research & Practice, USA; Mayo Clinic, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK) providing funding for the editorial process (http://ohg.cochrane.org/)
-
National Institute for Health Research (NIHR), UK
CRG funding acknowledgement: The NIHR is the largest single funder of the Cochrane Oral Health Group Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health
Declarations of interest
Paul Brocklehurst: no interests to declare. Omar Kujan: no interests to declare. Lucy A O'Malley: no interests to declare. Graham Ogden: no interests to declare. Simon Shepherd: no interests to declare. Anne‐Marie Glenny: no interests to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Sankaranarayanan 2000 {published data only}
- Ramadas K, Sankaranarayanan R, Jacob BJ, Thomas G, Somanathan T, Mahe C, et al. Interim results from a cluster randomized controlled oral cancer screening trial in Kerala, India. Oral Oncolgy 2003;39(6):580-8. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Mathew B, Jacob BJ, Thomas G, Somanathan T, Pisani P, et al. Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. Cancer 2000;88(3):664-73. [PubMed] [Google Scholar]
- Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Thomas G, Anju G, et al. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncology 2013;49(4):314-21. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet 2005;365(9475):1927-33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1998 {published data only}
- Allen CM. Toluidine blue: proceed with caution? Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 1998;86(3):255. [DOI] [PubMed] [Google Scholar]
Chamberlain 1993 {published data only}
- Chamberlain J. Evaluation of screening for cancer. Community Dental Health 1993;10 Suppl(1):5-11. [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen TH, Chiu YH, Luh DL, Yen MF, Wu HM, Chen LS, et al. Community-based multiple screening model: design, implementation, and analysis of 42,387 participants. Cancer 2004;100(8):1734-43. [DOI] [PubMed] [Google Scholar]
Cheng 2003 {published data only}
- Cheng B, Yang L. The clinical evaluation of Oratest in detecting oral mucosal lesions. Hua Xi Kou Qiang Yi Xue Za Zhi 2003;21(2):124-6. [PubMed] [Google Scholar]
Eliezri 1988 {published data only}
- Eliezri YD. The toluidine blue test: an aid in the diagnosis and treatment of early squamous cell carcinomas of mucous membranes. Journal of American Academy of Dermatology 1988;18(6):1339-49. [DOI] [PubMed] [Google Scholar]
Garrote 1995 {published data only}
- Frenández Garrote L, Sankaranarayanan R, Lence Anta JJ, Rodriguez Salvá A, Maxwell Parkin D. An evaluation of the oral cancer control program in Cuba. Epidemiology 1995;6(4):428-31. [DOI] [PubMed] [Google Scholar]
Gray 2000 {published data only}
- Gray M, Gold L, Elley A, Bursla A. The Effectiveness of Toluidine Blue Dye as Adjunct to Oral Cancer Screening in General Dental Practice. Birmingham: University of Birmingham, Department of Public Health & Epidemiology, 2000. [Google Scholar]
Gupta 1986 {published data only}
- Gupta PC, Mehta FS, Pindborg JJ, Aghi MB, Bhonsle RB, Daftery DK, et al. Intervention study for primary prevention of oral cancer among 36 000 Indian tobacco users. Lancet 1986;1(8492):1235-9. [DOI] [PubMed] [Google Scholar]
Gupta 1992 {published data only}
- Gupta PC, Mehta FS, Pindborg JJ, Bhonsle RB, Murti PR, Daftary DK, et al. Primary prevention trial of oral cancer in India: a 10-year follow-up study. Journal of Oral Pathology and Medicine 1992;21(10):433-9. [DOI] [PubMed] [Google Scholar]
Ikeda 1991 {published data only}
- Ikeda N, Ishii T, Iida S, Kawai T. Epidemiological study of oral leukoplakia based on mass screening for oral mucosal diseases in a selected Japanese population. Community Dentistry and Oral Epidemiology 1991;19(3):160-3. [DOI] [PubMed] [Google Scholar]
Ikeda 1995 {published data only}
- Ikeda N, Downer MC, Ishii T, Fukano H, Nagao T, Inoue K. Annual screening for oral cancer and precancer by invitation to 60-year-old residents of a city in Japan. Community Dental Health 1995;12(3):133-7. [PubMed] [Google Scholar]
Lavelle 2005 {published data only}
- Lavelle CL, Scully C. Criteria to rationalize population screening to control oral cancer. Oral Oncology 2005;41(1):11-6. [DOI] [PubMed] [Google Scholar]
Martin 1998 {published data only}
- Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 1998;85(4):444-6. [DOI] [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller RL, Simms BW, Gould AR. Toluidine blue staining for detection of oral premalignant lesions and carcinomas. Journal of Oral Pathology 1988;17(2):73-8. [DOI] [PubMed] [Google Scholar]
Moyer 1986 {published data only}
- Moyer GN, Taybos GM, Pelleu GB Jr. Toluidine blue rinse: potential for benign lesions in early detection of oral neoplasms. Journal of Oral Medicine 1986;41(2):111-3. [PubMed] [Google Scholar]
Mullhaupt 2004 {published data only}
- Mullhaupt B, Jenny D, Albert S, Schmid S, Fried M. Controlled prospective evaluation of the diagnostic yield of a laryngopharyngeal screening examination during upper gastrointestinal endoscopy. Gut 2004;53(9):1232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagao 2000 {published data only}
- Nagao T, Warnakulasuriya S, Ikeda N, Fukano H, Fujiwara K, Miyazaki H. Oral cancer screening as an integral part of general health screening in Tokoname City, Japan. Journal of Medical Screening 2000;7(4):203-8. [DOI] [PubMed] [Google Scholar]
Nagao 2000a {published data only}
- Nagao T, Ikeda N, Fukano H, Miyazaki H, Yano M, Warnakulasuriya S. Outcome following a population screening programme for oral cancer and precancer in Japan. Oral Oncology 2000;36(4):340-6. [DOI] [PubMed] [Google Scholar]
Nagao 2003 {published data only}
- Nagao T, Warnakulasuriya S. Annual screening for oral cancer detection. Cancer Detection and Prevention 2003;27(5):333-7. [DOI] [PubMed] [Google Scholar]
Patton 2003 {published data only}
- Patton LL. The effectiveness of community-based visual screening and utility of adjunctive diagnostic aids in the early detection of oral cancer. Oral Oncology 2003;39(7):708-23. [DOI] [PubMed] [Google Scholar]
Sankaranarayanan 1997 {published data only}
- Sankaranarayanan R. Health care auxiliaries in the detection and prevention of oral cancer. Oral Oncology 1997;33(3):149-54. [DOI] [PubMed] [Google Scholar]
Sankaranarayanan 2002 {published data only}
- Sankaranarayanan R, Fernandez Garrote L, Leuce Anta J, Pisani P, Rodriguez Salva A. Visual inspection in oral cancer screening in Cuba: a case-control study. Oral Oncology 2002;38(2):131-6. [DOI] [PubMed] [Google Scholar]
Silverman 1984 {published data only}
- Silverman S Jr, Migliorati C, Barbosa J. Toluidine blue staining in the detection of oral precancerous and malignant lesions. Oral Surgery, Oral Medicine, and Oral Pathology 1984;57(4):379-82. [DOI] [PubMed] [Google Scholar]
Su 2010 {published data only}
- Su WW, Yen AM, Chiu SY, Chen TH. A community-based RCT for oral cancer screening with toluidine blue. Journal of Dental Research 2010;89(9):933-7. [DOI] [PubMed] [Google Scholar]
Vahidy 1972 {published data only}
- Vahidy NA, Zaidi SH, Jafarey NA. Toluidine blue test for detection of carcinoma of the oral cavity: an evaluation. Journal of Surgical Oncology 1972;4(5):434-8. [DOI] [PubMed] [Google Scholar]
Zhang 2005 {published data only}
- Zhang L, Williams M, Poh CF, Laronde D, Epstein JB, Durham S, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Research 2005;65(17):8017-21. [DOI] [PubMed] [Google Scholar]
Additional references
American 1992
- American Cancer Society. Update January 1992: The American Cancer Society guidelines for the cancer-related checkup. CA: a Cancer Journal for Clinicians 1992;42:44-5. [DOI] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637-9. [DOI] [PubMed] [Google Scholar]
Blot 1988
- Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Research 1988;48(11):3282-7. [PubMed] [Google Scholar]
Boyle 2005
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Annals of Oncology 2005;16(3):481-8. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2010
- Brocklehurst PR, Baker SR, Speight PM. A qualitative study examining the experience of primary care dentists in the detection and management of potentially malignant lesions. 2. Mechanics of the referral and patient communication. British Dental Journal 2010;208(2):E4. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2010a
- Brocklehurst PR, Baker SR, Speight PM. Oral cancer screening: what have we learnt and what is there still to achieve? Future Oncology 2010;6(2):299-304. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2010b
- Brocklehurst PR, Baker SR, Speight PM. Factors which determine the referral of potentially malignant disorders by primary care dentists. Journal of Dentistry 2010;38(7):569-78. [DOI] [PubMed] [Google Scholar]
Cancer Research UK
- Cancer Research UK. http://info.cancerresearchuk.org/cancerstats/types/oral (accessed 14 April 2010).
Clarkson 2003
- Clarkson J, Harrison JE, Ismail AI, Needleman I, Worthington H. Evidence Based Dentistry for Effective Practice. London: Taylor & Francis Group, 2003. [Google Scholar]
Cleveland 2011
- Cleveland JL, Junger ML, Saraiya M, Markowitz LE, Dunne EF, Epstein JB. The connection between human papillomavirus and oropharyngeal squamous cell carcinomas in the United States: implications for dentistry. Journal of the American Dental Association 2011;142(8):915-24. [DOI] [PubMed] [Google Scholar]
Conway 2006
- Conway DI, Stockton DL, Warnakulasuriya KA, Ogden G, Macpherson LM. Incidence of oral and oropharyngeal cancer in United Kingdom (1990-1999) - recent trends and regional variation. Oral Oncology 2006;42(6):586-92. [DOI] [PubMed] [Google Scholar]
Conway 2010a
- Conway DI, McKinney PA, McMahon AD, Ahrens W, Schmeisser N, Benhamou S, et al. Socioeconomic factors associated with risk of upper aerodigestive tract cancer in Europe. European Journal of Cancer 2010;46(3):588-98. [DOI] [PubMed] [Google Scholar]
Conway 2010b
- Conway DI, McMahon AD, Smith K, Black R, Robertson G, Devine J, et al. Components of socioeconomic risk associated with head and neck cancer: a population-based case-control study in Scotland. The British Journal of Oral and Maxillofacial Surgery 2010;48(1):11-7. [DOI] [PubMed] [Google Scholar]
Day 1992
- Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer 1992;70(1):14-9. [DOI] [PubMed] [Google Scholar]
Diniz‐Freitas 2004
- Diniz-Freitas M, Garcia-Garcia A, Crespo-Abelleira A, Martins-Carneiro JL, Gandara-Rey JM. Applications of exfoliative cytology in the diagnosis of oral cancer. Medicina Oral 2004;9(4):355-61. [PubMed] [Google Scholar]
Doobaree 2009
- Doobaree IU, Landis SH, Linklater KM, El-Hariry I, Moller H, Tyczynski J. Head and neck cancer in South East England between 1995-1999 and 2000-2004: An estimation of incidence and distribution by site, stage and histological type. Oral Oncology 2009;45(9):809-14. [DOI] [PubMed] [Google Scholar]
Downer 1998
- Downer MC, Jullien JA, Speight PM. An interim determination of health gain from oral cancer and precancer screening: 3. Preselecting high risk individuals. Community Dental Health 1998;15(2):72-6. [PubMed] [Google Scholar]
Downer 2004
- Downer MC, Moles DR, Palmer S, Speight PM. A systematic review of test performance in screening for oral cancer and precancer. Oral Oncology 2004;40(3):264-73. [DOI] [PubMed] [Google Scholar]
Duffy 2001
- Duffy S, Hill C, Esteve J. Quantitative Methods for the Evaluation of Cancer Screening. London: Arnold, 2001. [Google Scholar]
Duffy 2005
- Duffy SW, Agbaje O, Tabar L, Vitak B, Bjurstam N, Bjorneld L, et al. Overdiagnosis and overtreatment of breast cancer: estimates of overdiagnosis from two trials of mammographic screening for breast cancer. Breast Cancer Research 2005;7(6):258-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 1993
- Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S. Report of International Workshop on Screening for Breast Cancer. Journal of the National Cancer Institute 1993;85(20):1644-56. [DOI] [PubMed] [Google Scholar]
Gøtzsche 2006
- Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD001877. [DOI: 10.1002/14651858.CD001877.pub2] [DOI] [PubMed] [Google Scholar]
Gøtzsche 2013
- Gøtzsche, PC Jørgensen, KJ. Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD001877. [DOI: 10.1002/14651858.CD001877.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauck 1991
- Hauck WW, Gilliss CL, Donner A, Gortner S. Randomization by cluster. Nursing Research 1991;40(6):356-8. [PubMed] [Google Scholar]
Hewitson 2007
- Hewitson P, Glasziou PP, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD001216. [DOI: 10.1002/14651858.CD001216.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hindle 2000
- Hindle I, Downer MC, Moles DR, Speight PM. Is alcohol responsible for more intra-oral cancer? Oral Oncology 2000;36(4):328-33. [DOI] [PubMed] [Google Scholar]
Holmstrup 2007
- Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Oral premalignant lesions: is a biopsy reliable? Journal of Oral Pathology and Medicine 2007;36:262-6. [DOI] [PubMed] [Google Scholar]
Holmstrup 2009
- Holmstrup P. Can we prevent malignancy by treating premalignant lesions? Oral Oncology 2009;45:549-50. [DOI] [PubMed] [Google Scholar]
IARC 2010
- International Agency for Research on Cancer (IARC), World Health Organization. Cancer Incidence in Five Continents CI5, vol IX. http://ci.iarc.fr/ (accessed 15 April 2010).
Kaplan 2005
- Kaplan RM. Screening for cancer: are resources being used wisely? Recent Results in Cancer Research 2005;166:315-34. [DOI] [PubMed] [Google Scholar]
Kay 1991
- Kay BR, Witte DL. The impact of cancer biology, lead time bias, and length bias in the debate about cancer screening tests. Journal of Insurance Medicine 1991;23(2):102-4. [PubMed] [Google Scholar]
Kujan 2005
- Kujan O, Glenny AM, Duxbury AJ, Thakker N, Sloan P. Evaluation of screening strategies for improving oral cancer mortality: a Cochrane systematic review. Journal of Dental Education 2005;69(2):255-65. [PubMed] [Google Scholar]
La Vecchia 1997
- La Vecchia C, Tavani A, Franceschi S, Levi F, Corrao G, Negri E. Epidemiology and prevention of oral cancer. Oral Oncology 1997;33(5):302-12. [DOI] [PubMed] [Google Scholar]
La Vecchia 2004
- La Vecchia C, Lucchini F, Negri E, Levi F. Trends in oral cancer mortality in Europe. Oral Oncology 2004;40(4):433-9. [DOI] [PubMed] [Google Scholar]
Lim 2003
- Lim K, Moles DR, Downer MC, Speight PM. Opportunistic screening for oral cancer and precancer in general dental practice: results of a demonstration study. British Dental Journal 2003;194(9):497-502. [DOI] [PubMed] [Google Scholar]
Lingen 2008
- Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncology 2008;44(1):10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lippman 1989
- Lippman SM, Hong WK. Second malignant tumors in head and neck squamous cell carcinoma: the overshadowing threat for patients with early-stage disease. International Journal of Radiation Oncology, Biology, Physics 1989;17(3):691-4. [DOI] [PubMed] [Google Scholar]
McLeod 2005
Ministry of Health 2005
- Ministry of Health. National Cancer Control Programme, Sri Lanka. Cancer Incidence Data: Sri Lanka Year 2000. 6th Publication. Maharagama: NCCP, 2005. [Google Scholar]
Napier 2008
- Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. Journal of Oral Pathology and Medicine 2008;37(1):1-10. [DOI] [PubMed] [Google Scholar]
Noonan 2005
- Noonan VL, Kabani S. Diagnosis and management of suspicious lesions of the oral cavity. Otolaryngologic Clinics of North America 2005;38(1):21-35. [DOI] [PubMed] [Google Scholar]
NSC 2010
- NSC. National Screening Committee. Available at http://www.screening.nhs.uk/screening (accessed 14 April 2010).
Ogden 1998
- Ogden GR, Wight AJ. Aetiology of oral cancer: alcohol. The British Journal of Oral and Maxillofacial Surgery 1998;36(4):247-51. [DOI] [PubMed] [Google Scholar]
Ogden 2005
- Ogden GR. Alcohol and oral cancer. Alcohol 2005;35(3):169-73. [DOI] [PubMed] [Google Scholar]
Onizawa 2003
- Onizawa K, Nishihara K, Yamagata K, Yusa H, Yanagawa T, Yoshida H. Factors associated with diagnostic delay of oral squamous cell carcinoma. Oral Oncology 2003;39(8):781-8. [DOI] [PubMed] [Google Scholar]
Paci 2004
- Paci E, Warwick J, Falini P, Duffy SW. Overdiagnosis in screening: is the increase in breast cancer incidence rates a cause for concern? Journal of Medical Screening 2004;11(1):23-7. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a Cancer Journal for Clinicians 2005;55(2):74-108. [DOI] [PubMed] [Google Scholar]
Patton 2008
- Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. Journal of the American Dental Association 2008;139(7):896-905. [DOI] [PubMed] [Google Scholar]
Petersen 2009
- Petersen PE. Oral cancer prevention and control - the approach of the World Health Organization. Oral Oncology 2009;45(4-5):454-60. [DOI] [PubMed] [Google Scholar]
Petti 2003
- Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncology 2003;39(8):770-80. [DOI] [PubMed] [Google Scholar]
Petti 2005
- Petti S, Scully C. Oral cancer: the association between nation-based alcohol-drinking profiles and oral cancer mortality. Oral Oncology 2005;41(8):828-34. [DOI] [PubMed] [Google Scholar]
Radoï 2013
- Radoï L, Paget-Bailly S, Cyr D, Papadopoulos A, Guida F, Schmaus A, et al. Tobacco smoking, alcohol drinking and risk of oral cavity cancer by subsite: results of a French population-based case-control study, the ICARE study. European Journal of Cancer Prevention 2013;22(3):268-76. [DOI] [PubMed] [Google Scholar]
Rogers 2009
- Rogers SN, Brown JS, Woolgar JA, Lowe D, Magennis P, Shaw RJ, et al. Survival following primary surgery for oral cancer. Oral Oncology 2009;45(3):201-11. [DOI] [PubMed] [Google Scholar]
Rusthoven 2010
- Rusthoven KE, Raben D, Song JI, Kane M, Altoos TA, Chen C. Survival and patterns of relapse in patients with oral tongue cancer. Journal of Oral and Maxillofacial Surgery 2010;68(3):584-9. [DOI] [PubMed] [Google Scholar]
Salaspuro 2011
- Salaspuro M. Acetaldehyde and gastric cancer. Journal of Digestive Diseases 2011;12(2):51-9. [DOI] [PubMed] [Google Scholar]
Sanders 2011
- Sanders AE, Slade GD, Patton LL. National prevalence of oral HPV infection and related risk factors in the U.S. adult population. Oral Diseases 2011;18(5):430-41. [DOI] [PubMed] [Google Scholar]
Scully 2009
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncology 2009;45(4-5):301-8. [DOI] [PubMed] [Google Scholar]
SEER 2010
- National Cancer Institute. SEER Cancer Statistics Review 1975-2004. http://seer.cancer.gov/statfacts/html/oralcav.html (accessed 14 April 2010).
Speight 1992
- Speight PM, Zakrzewska J, Downer MC. Screening for oral cancer and precancer. European Journal of Cancer 1992;28B(1):45-8. [DOI] [PubMed] [Google Scholar]
Speight 2006
- Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al. The cost-effectiveness of screening for oral cancer in primary care. Health Technology Assessment 2006;10(14):1-144. [DOI] [PubMed] [Google Scholar]
Stewart 2003
- Stewart BW, Kleihues P International Agency for Research on Cancer. World Cancer Report. Lyon: IARC Press, 2003. [DOI] [PubMed] [Google Scholar]
Subramanian 2009
- Subramanian S, Sankaranarayanan R, Bapat B, Somanathan T, Thomas G, Mathew B, et al. Cost-effectiveness of oral cancer screening: results from a cluster randomized controlled trial in India. Bulletin of the World Health Organization 2009;87(3):200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarvainen 2004
- Tarvainen L, Suuronen R, Lindqvist C, Malila N. Is the incidence of oral and pharyngeal cancer increasing in Finland? An epidemiological study of 17,383 cases in 1953-1999. Oral Diseases 2004;10(3):167-72. [DOI] [PubMed] [Google Scholar]
van der Waal 2009
- Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncology 2009;45(4-5):317-23. [DOI] [PubMed] [Google Scholar]
Walsh 2013
- Walsh T, Liu JLY, Brocklehurst P, Glenny A-M, Lingen M, Kerr AR et al. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD010173. [DOI: 10.1002/14651858.CD010173.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warnakulasuriya 2007
- Warnakulasuriya S, Johnson NW, Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. Journal of Oral Pathology and Medicine 2007;36(10):575-80. [DOI] [PubMed] [Google Scholar]
Warnakulasuriya 2009
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncology 2009;45(4-5):309-16. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization (WHO) Commission on Macroeconomics and Health. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: WHO, 2001. [Google Scholar]
Wilson 1968
- Wilson JM, Jungner YG. Principles and Practice of Screening for Disease. Public Health Paper Number 34. Geneva: WHO, 1968. [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision-making. Evidence-based Dentistry 2015;16(3):69-71. [DOI] [PubMed] [Google Scholar]
Yellowitz 2000
- Yellowitz JA, Horowitz AM, Drury TF, Goodman HS. Survey of U.S. dentist's knowledge and opinions about oral pharyngeal cancer. Journal of the American Dental Association 2000;131(5):653-61. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brocklehurst 2010c
- Brocklehurst P, Kujan O, Glenny A-M, Oliver R, Sloan P, Ogden G, Shepherd S. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database of Systematic Reviews 2010, Issue 11. Art. No: CD004150. [DOI: 10.1002/14651858.CD004150.pub3] [DOI] [PubMed] [Google Scholar]
Kujan 2003
- Kujan O, Glenny AM, Duxbury AJ, Thakker N, Sloan P. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD004150. [DOI: 10.1002/14651858.CD004150] [DOI] [PubMed] [Google Scholar]
Kujan 2006
- Kujan O, Glenny AM, Oliver R, Thakker N, Sloan P. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD004150. [DOI: 10.1002/14651858.CD004150.pub2] [DOI] [PubMed] [Google Scholar]



