Abstract
Background
Sore throat is a common reason for people to present for medical care. Although it remits spontaneously, primary care doctors commonly prescribe antibiotics for it.
Objectives
To assess the benefits of antibiotics for sore throat for patients in primary care settings.
Search methods
We searched CENTRAL 2013, Issue 6, MEDLINE (January 1966 to July week 1, 2013) and EMBASE (January 1990 to July 2013).
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs of antibiotics versus control assessing typical sore throat symptoms or complications.
Data collection and analysis
Two review authors independently screened studies for inclusion and extracted data. We resolved differences in opinion by discussion. We contacted trial authors from three studies for additional information.
Main results
We included 27 trials with 12,835 cases of sore throat. We did not identify any new trials in this 2013 update.
1. Symptoms Throat soreness and fever were reduced by about half by using antibiotics. The greatest difference was seen at day three. The number needed to treat to benefit (NNTB) to prevent one sore throat at day three was less than six; at week one it was 21.
2. Non‐suppurative complications The trend was antibiotics protecting against acute glomerulonephritis but there were too few cases to be sure. Several studies found antibiotics reduced acute rheumatic fever by more than two‐thirds within one month (risk ratio (RR) 0.27; 95% confidence interval (CI) 0.12 to 0.60).
3. Suppurative complications Antibiotics reduced the incidence of acute otitis media within 14 days (RR 0.30; 95% CI 0.15 to 0.58); acute sinusitis within 14 days (RR 0.48; 95% CI 0.08 to 2.76); and quinsy within two months (RR 0.15; 95% CI 0.05 to 0.47) compared to those taking placebo.
4. Subgroup analyses of symptom reduction Antibiotics were more effective against symptoms at day three (RR 0.58; 95% CI 0.48 to 0.71) if throat swabs were positive for Streptococcus, compared to RR 0.78; 95% CI 0.63 to 0.97 if negative. Similarly at week one the RR was 0.29 (95% CI 0.12 to 0.70) for positive and 0.73 (95% CI 0.50 to 1.07) for negative Streptococcus swabs.
Authors' conclusions
Antibiotics confer relative benefits in the treatment of sore throat. However, the absolute benefits are modest. Protecting sore throat sufferers against suppurative and non‐suppurative complications in high‐income countries requires treating many with antibiotics for one to benefit. This NNTB may be lower in low‐income countries. Antibiotics shorten the duration of symptoms by about 16 hours overall.
Plain language summary
Antibiotics for people with sore throats
Question
This review sought to determine whether antibiotics are effective for treating the symptoms and reducing the potential complications associated with sore throats.
Background
Sore throats are infections caused by bacteria or viruses. People usually recover quickly (usually after three or four days), although some develop complications. A serious but rare complication is rheumatic fever, which affects the heart and joints. Antibiotics reduce bacterial infections but they can cause diarrhea, rash and other adverse effects and communities build resistance to them.
Study characteristics
The review is current to July 2013 and included 27 trials with 12,835 cases of sore throat. All of the included studies were randomised, placebo‐controlled trials which sought to determine if antibiotics helped reduce symptoms of either sore throat, fever and headache or the occurrence of more serious complications. Studies were conducted among both children and adults.
Key results
The review found that antibiotics shorten the duration of pain symptoms by an average of about one day and can reduce the chance of rheumatic fever by more than two‐thirds in communities where this complication is common. Other complications associated with sore throat are also reduced through antibiotic use.
Quality of evidence
The quality of the included studies was moderate to high. However, there were very few recent trials included in the review (only three since 2000), hence it is unclear if changes in bacterial resistance in the community may have affected the effectiveness of antibiotics.
Summary of findings
for the main comparison.
| Antibiotics compared with placebo for sore throat | ||||||
|
Patient or population: patients presenting with sore throat Settings: community Intervention: antibiotics Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Corresponding risk | Assumed risk | |||||
| Antibiotics | Placebo | |||||
| Sore throat: day 3 | 0.66 | 0.72 | 0.68 to 0.76 | 3621 (15) | High | |
| Sore throat: day 7 | 0.18 | 0.65 | 0.55 to 0.76 | 2974 (13) | High | |
| Rheumatic fever | 0.017 | 0.29 | 0.18 to 0.44 | 10,101 (16) | High | Based largely on risk in pre‐1960 trials |
| Glomerulonephritis | 0.001 | 0.22 | 0.07 to 1.32 | 5147 (10) | Low | Sparse data: 2 cases only |
| Quinsy | 0.023 | 0.14 | 0.05 to 0.39 | 2433 (8) | High | |
| Otitis media | 0.02 | 0.28 | 0.15 to 0.52 | 3760 (11) | High | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Sore throat is a very common reason for people to attend primary care settings (ABS 1985). Moreover, four to six times as many people suffering sore throat do not seek care (Goslings 1963; Horder 1954). Sore throat is a disease that remits spontaneously, that is, 'cure' is not dependent on treatment (Del Mar 1992c). Nonetheless, primary care doctors commonly prescribe antibiotics for sore throat and other upper respiratory tract infections. There are large differences in clinical practice between countries (Froom 1990) and between primary care doctors (Howie 1971).
Description of the intervention
The administration of antibiotics is likely to shorten the time to the remittance of symptoms and reduce the likelihood of complications in patients whose sore throat has a bacteriological aetiology (van Driel 2013). However, their benefits may be limited in the treatment of sore throat more generally (Reveiz 2013). Traditionally, doctors have attempted to decide whether the cause of the infection is bacterial, especially when caused by the group A beta‐haemolytic Streptococcus (GABHS) (which can cause acute rheumatic fever and acute glomerulonephritis). However, deciding the aetiological agent is difficult (Del Mar 1992b).
How the intervention might work
Antibiotics target bacteria which are potentially responsible for sore throat symptoms and possible subsequent suppurative and non‐suppurative sequelae. Successful eradication of bacteria may promote faster healing and prevention of secondary complications. However, not all sore throat cases are of bacteriologic origin and bacteria may resist antibiotic treatment which could limit the overall effectiveness of the intervention.
Why it is important to do this review
Whether or not to prescribe antibiotics for sore throat is controversial. The issue is important because it is a very common disease and differences in prescribing result in large cost differences. Moreover, increased prescribing increases patient attendance rates (Howie 1978; Little 1997). This review is built on an early meta‐analysis (Del Mar 1992a) and is an update of previous Cochrane Reviews (Del Mar 1997; Del Mar 2000; Del Mar 2004; Del Mar 2006; Spinks 2009).
Objectives
To assess the benefits of antibiotics for sore throat for patients in primary care settings.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Patients presenting to primary care facilities with symptoms of sore throat.
Types of interventions
Antibiotics or placebo control.
Types of outcome measures
Primary outcomes
Symptoms of sore throat on day three.
Symptoms of sore throat at one week (days six to eight).
Secondary outcomes
Symptoms of fever at day three.
Symptoms of headache at day three.
-
Incidence of suppurative complications:
quinsy;
acute otitis media;
acute sinusitis.
-
Incidence of non‐suppurative complications:
incidence of acute rheumatic fever within two months;
acute glomerulonephritis within one month.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 6, part of The Cochrane Library,www.thecochranelibrary.com (accessed 11 July 2013), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (May 2011 to July week 1, 2013) and EMBASE (May 2011 to July 2013). See Appendix 1 for details of previous searches.
MEDLINE and CENTRAL were searched using the search strategy shown below. We combined the MEDLINE search string with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity and precision‐maximising version (2008 revision) (Lefebvre 2011). We adapted the search string for EMBASE (Appendix 2). There were no language or publication restrictions.
MEDLINE (Ovid)
1 exp Pharyngitis/ 2 pharyngit*.tw. 3 exp Nasopharyngitis/ 4 (nasopharyngit* or rhinopharyngit*).tw. 5 exp Tonsillitis/ 6 tonsillit*.tw. 7 (tonsil* adj2 (inflam* or infect*)).tw. 8 ((throat* or pharyn*) adj3 (infect* or inflam* or strep*)).tw. 9 (sore* adj2 throat*).tw. 10 or/1‐9 11 exp Anti‐Bacterial Agents/ 12 antibiot*.tw,nm. 13 (azithromycin* or clarithromycin* or erythromycin* or roxithromycin* or macrolide* or cefamandole* or cefoperazone* or cefazolin* or cefonicid* or cefsulodin* or cephacetrile* or cefotaxime* or cephalothin* or cephapirin* or cephalexin* or cephaclor* or cephadroxil* or cephaloglycin* or cephradine* or cephaloridine* or ceftazidime* or cephamycin* or cefmetazole* or cefotetan* or cefoxitin* or cephalosporin* or cefpodoxime* or cefuroxime* or cefixime* or amoxicillin* or amoxycillin* or ampicillin* or sulbactum* or tetracyclin* or clindamycin* or lincomycin* or doxycyclin* or fluoroquinolone* or ciprofloxacin* or fleroxacin* or enoxacin* or norfloxacin* or ofloxacin* or pefloxacin* or moxifloxacin* or esparfloxacin* or clindamicin* or penicillin* or ticarcillin* or beta‐lactam* or levofloxacin* or trimethoprim* or co‐trimoxazole).tw,nm. 14 or/11‐13 15 10 and 14
Searching other resources
We searched ClinicalTrials.gov and WHO ICTRP (11 July 2013) for completed and ongoing trials. We hand‐checked references of selected studies and relevant reviews to find additional studies.
Data collection and analysis
Selection of studies
Two review authors (AS, CD) independently screened abstracts of potential studies and retrieved full articles for those that were trials. Two review authors (AS, CD) examined the full articles and either selected for inclusion or rejected to the excluded studies list. We resolved differences in opinion by discussion.
Data extraction and management
Two review authors (AS, CDM) independently extracted data from the included studies based on patient‐relevant outcomes: namely the complications and symptoms listed above. Data extraction involved reading from tables, graphs and, in some cases, contacting trial authors for raw data (Dagnelie 1996; Little 1997; Zwart 2000; Zwart 2003).
Assessment of risk of bias in included studies
We assessed risk of bias according to the approach indicated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the following six criteria: adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias.
Measures of treatment effect
All treatment effect outcomes were dichotomous data, reported as risk ratios (RR). We reported occurrence of complications during the study period for suppurative and non‐suppurative complications. We assessed the presence of symptoms (sore throat, fever, headache) when possible at day three and week one (days six to eight). We also calculated numbers needed to treat to benefit (NNTB) for the primary outcomes.
Dealing with missing data
We performed an intention‐to treat (ITT) analysis for all outcomes.
Assessment of heterogeneity
We assessed heterogeneity by using the Chi2 test with the significance level set at 0.1. We determined the effect of heterogeneity by the I2 statistic which indicates the proportion of total variability which can be explained by heterogeneity. We interpreted values of the I2 statistic greater than 50% as indicating substantial heterogeneity, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We combined data where possible in order to perform meta‐analyses to report RR for all relevant outcomes. We used a random‐effects meta‐analytical method (Mantel‐Haenszel) in order to account for heterogeneity that was detected using the methods described above. Not all studies were able to contribute data to each of the meta‐analyses performed.
Subgroup analysis and investigation of heterogeneity
We performed a series of subgroup analyses to assess the differences in outcomes across various subgroups within the participant population:
treatment with penicillin (omitting other antibiotics);
children compared with adults;
positive throat swab versus negative throat swab versus untested and/or inseparable data for group A beta‐haemolytic Streptococcus (GABHS).
Sensitivity analysis
We performed sensitivity analyses to assess the degree to which results were influenced by the following criteria:
early (pre‐1975) versus later (post‐1975) studies;
blinded versus unblinded studies;
antipyretics administered versus no antipyretics administered.
Results
Description of studies
Results of the search
A total of 61 studies were considered for the review. Of these, there were 27 controlled studies that met the inclusion criteria and were included in the review. There were no new trials included in this 2013 update. However, three new trials were considered and subsequently excluded.
Included studies
The included studies investigated a total of 12,835 cases of sore throat. The majority of studies were conducted in the 1950s, during which time the rates of serious complications (especially acute rheumatic fever) were much higher than today. Seven studies published in the last 15 years (between 1996 to 2003) were included. However, no new studies have been published since 2003.
The age of participants ranged from less than one year to older than 50 years. The participants of eight early studies were young male recruits from the United States Air Force (Brink 1951; Brumfitt 1957; Catanzaro 1954; Chamovitz 1954; Denny 1950; Denny 1953; MacDonald 1951; Wannamaker 1951). Seven of the remaining studies recruited children up to 18 years of age only (El‐Daher 1991; Krober 1985; Nelson 1984; Pichichero 1987; Siegel 1961; Taylor 1977; Zwart 2000), three recruited only adults or adolescents aged 15 years or over (Howe 1997; Petersen 1997; Zwart 2003) and nine studies recruited both adults and children (Bennike 1951; Chapple 1956; Dagnelie 1996; De Meyere 1992; Landsman 1951; Leelarasamee 2000; Little 1997; Middleton 1988; Whitfield 1981).
All studies recruited patients presenting with symptoms of sore throat. The majority of studies did not distinguish between bacterial and viral aetiology. However, seven studies included or analyzed results for group A beta haemolytic Streptococcus (GABHS) positive patients only (Catanzaro 1954; De Meyere 1992; El‐Daher 1991; Krober 1985; Middleton 1988; Nelson 1984; Pichichero 1987), one study distinguished differences in outcomes between GABHS‐positive and negative patients (Dagnelie 1996) and two studies specifically excluded patients who were GABHS‐positive (Petersen 1997; Taylor 1977).
Excluded studies
The most common reason for exclusion was lack of appropriate control group (n = 13). Other reasons for exclusion were: irrelevant or non‐patient centred outcomes (n = 6), main complaint other than acute sore throat (n = 6), inappropriate or no randomisation to treatment (n = 5), an intervention other than antibiotics was being tested (n = 2), the study tracked natural course of illness only (n = 1) or that the study reported previously published data already included (n = 1).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
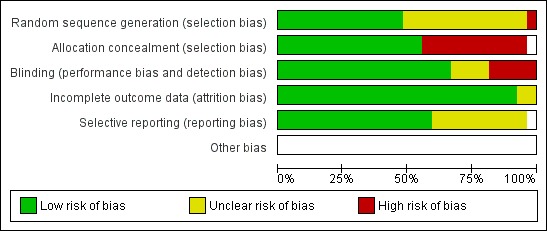
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
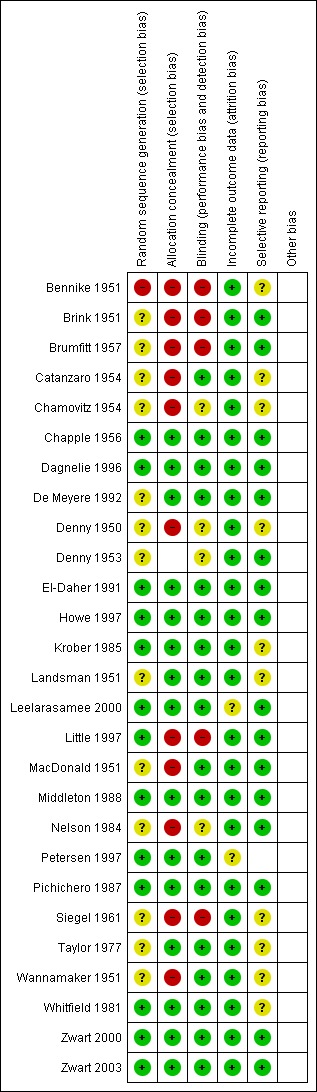
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In most early studies, participants were randomised to treatment and control groups by methods that could potentially introduce bias (for example, Air Force serial number, drawing a card from a deck, hospital bed number) or not randomised at all. Allocation methods were generally appropriate in the later studies.
Blinding
Eighteen of the studies were double‐blinded and three were single‐blinded.
Incomplete outcome data
Outcome data were complete for nearly all studies. For one study it was not clear how many participants maintained pain score diaries and some participants who were initially randomised were excluded due to being GABHS‐positive (Petersen 1997).
Other potential sources of bias
The use of antipyretic analgesics was not stated in nine studies, administered routinely in five studies and prohibited in four studies. The prohibition of analgesics might exaggerate any small symptomatic benefit of antibiotics over control if antipyretic analgesics are usually recommended in normal practice.
Effects of interventions
See: Table 1
Primary outcomes
1. Symptoms of sore throat on day three
At day three of the illness, antibiotics reduced symptoms of sore throat (risk ratio (RR) 0.68; 95% confidence interval (CI) 0.59 to 0.79) (Analysis 1.1). Day three was the greatest time of benefit because the symptoms of only half the participants had settled.
1.1. Analysis.
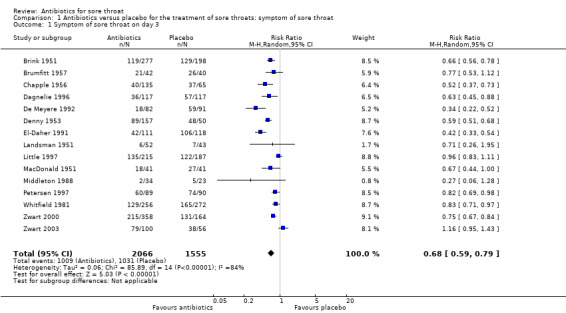
Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 1 Symptom of sore throat on day 3.
2. Symptoms of sore throat at one week (days six to eight)
At one week (six to eight days) the RR of experiencing sore throat was 0.49 (95% CI 0.32 to 0.76) (Analysis 1.5), although 82% of controls were better by this time.
1.5. Analysis.
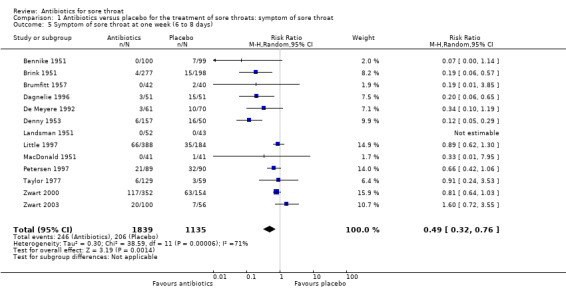
Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 5 Symptom of sore throat at one week (6 to 8 days).
Secondary outcomes
1. Symptoms of fever at day three
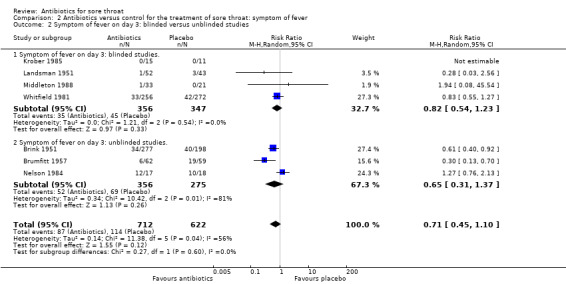
At day three of the illness, antibiotics reduced symptoms of fever (RR 0.71; 95% CI 0.45 to 1.10) (Analysis 2.1).
2.1. Analysis.
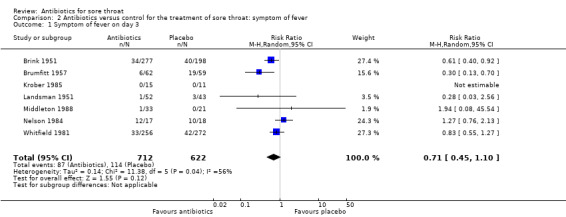
Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 1 Symptom of fever on day 3.
2. Symptoms of headache at day three
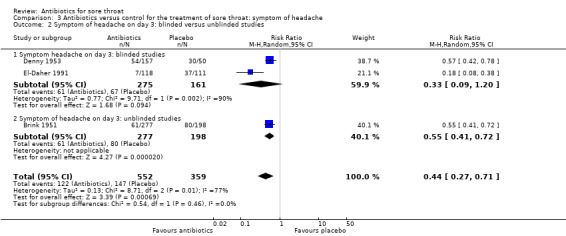
At day three of the illness, antibiotics reduced symptoms of headache (RR 0.44; 95% CI 0.27 to 0.71) (Analysis 3.1).
3.1. Analysis.
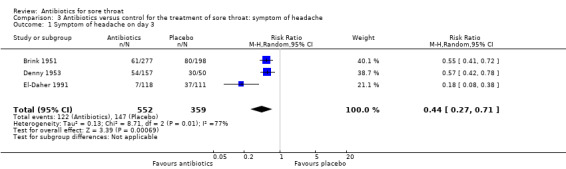
Comparison 3 Antibiotics versus control for the treatment of sore throat: symptom of headache, Outcome 1 Symptom of headache on day 3.
3. Incidence of suppurative complications
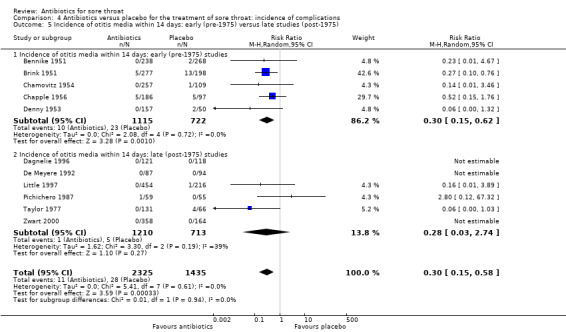
Antibiotics reduced the incidence of acute otitis media to about one‐third of that in the placebo group (RR 0.30; 95% CI 0.15 to 0.58) (Analysis 4.4) and reduced the incidence of acute sinusitis to about one‐half of that in the placebo group (RR 0.48; 95% CI 0.08 to 2.76) (Analysis 4.6). Data indicate that the incidence of quinsy was also reduced in relation to the placebo group (RR 0.15; 95% CI 0.05 to 0.47) (Analysis 4.7).
4.4. Analysis.
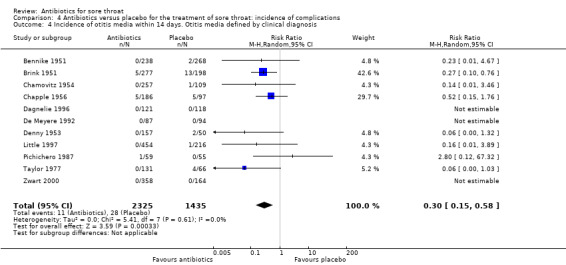
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 4 Incidence of otitis media within 14 days. Otitis media defined by clinical diagnosis.
4.6. Analysis.
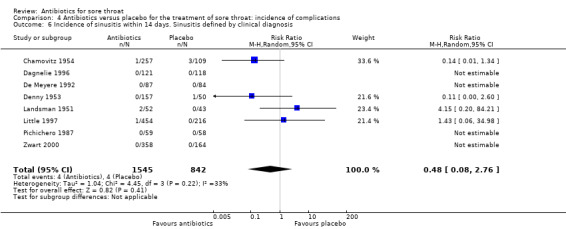
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 6 Incidence of sinusitis within 14 days. Sinusitis defined by clinical diagnosis.
4.7. Analysis.
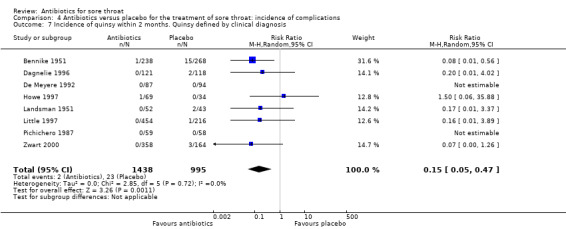
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 7 Incidence of quinsy within 2 months. Quinsy defined by clinical diagnosis.
4. Incidence of non‐suppurative complications
Cases of acute glomerulonephritis only occurred in the control group which suggests protection by antibiotics. However, there were only two cases and only 10 studies reported on acute glomerulonephritis as an endpoint. Therefore, our estimate of the protection has a very wide 95% CI (RR 0.22; 95% CI 0.02 to 2.08) (Analysis 4.8) which precludes us from definitively claiming that antibiotics protect sore throat sufferers from acute glomerulonephritis.
4.8. Analysis.
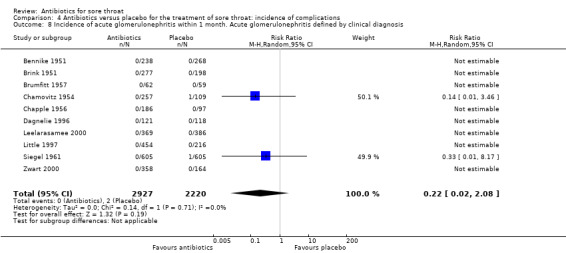
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 8 Incidence of acute glomerulonephritis within 1 month. Acute glomerulonephritis defined by clinical diagnosis.
Several studies found benefit from antibiotics for acute rheumatic fever which reduced this complication to about one‐quarter of that in the placebo group (RR 0.27; 95% CI 0.12 to 0.60) (Analysis 4.1). Few studies examined antibiotics other than penicillin. Confining the analysis to penicillin alone resulted in no difference in estimated protection (RR 0.27; 95% CI 0.14 to 0.50) (Analysis 4.2).
4.1. Analysis.
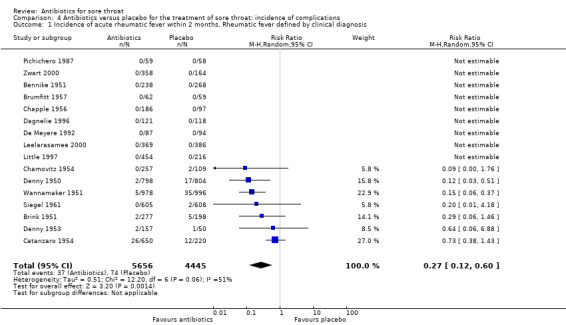
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 1 Incidence of acute rheumatic fever within 2 months. Rheumatic fever defined by clinical diagnosis.
4.2. Analysis.

Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 2 Incidence of acute rheumatic fever within 2 months. Penicillin versus placebo.
Subgroup analysis of symptom reduction
1. Blind versus unblinded studies
There was no significant difference between blinded and unblinded studies for symptoms of sore throat at day three (RR 0.65; 95% CI 0.54 to 0.78 and RR 0.79; 95% CI 0.60 to 1.05, respectively) (Analysis 1.2) nor at one week (RR 0.62; 95% CI 0.38 to 1.03 and RR 0.30; 95% CI 0.08 to 1.15, respectively) (Analysis 1.6). Contrary to expectation, the trend was for a greater effect of antibiotics for blind studies at day three.
1.2. Analysis.
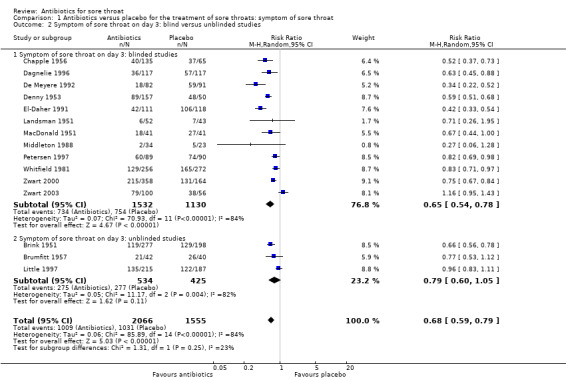
Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 2 Symptom of sore throat on day 3: blind versus unblinded studies.
1.6. Analysis.

Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 6 Symptom of sore throat at one week (6 to 8 days): blind versus unblinded studies.
2. Antipyretics administered versus not administered
Use of antipyretics led to no significant difference between studies in which antipyretics were offered and those in which they were not (RR 0.52; 95% CI 0.33 to 0.81 and RR 0.62; 95% CI 0.55 to 0.70, respectively) (Analysis 1.3).
1.3. Analysis.
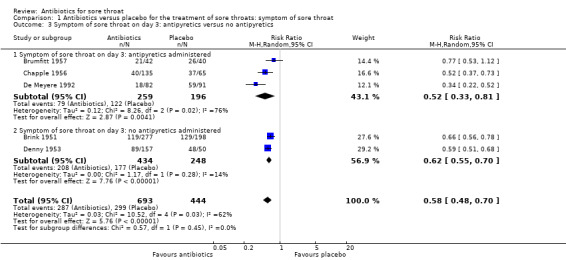
Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 3 Symptom of sore throat on day 3: antipyretics versus no antipyretics.
3. Throat swabs positive for Streptococcus versus negative for Streptococcus versus not tested and/or inseparable combined data
The probability of still experiencing pain on day three is slightly more than one‐half (RR 0.58; 95% CI 0.48 to 0.71) for those participants who had positive throat swabs for GABHS, compared to three‐quarters (RR 0.78; 95% CI 0.63 to 0.97) for those with negative swabs (Analysis 1.4). There was a similar effect at one week (RR 0.29; 95% CI 0.12 to 0.70 and RR 0.73; 95% CI 0.50 to 1.07, respectively) (Analysis 1.7). That is, the effectiveness of antibiotics is increased in people with Streptococci growing in the throat.
1.4. Analysis.

Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 4 Symptom of sore throat on day 3: GABHS‐positive throat swab, negative swab, untested/inseparable.
1.7. Analysis.
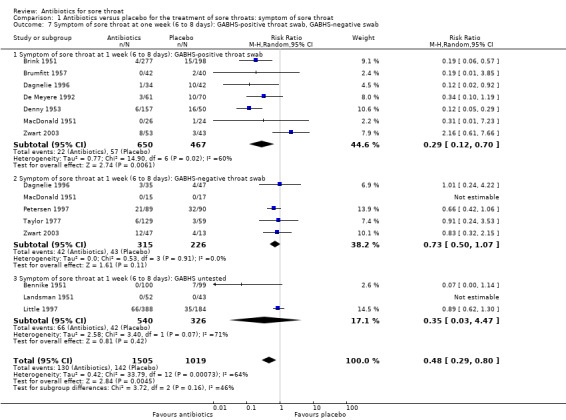
Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 7 Symptom of sore throat at one week (6 to 8 days): GABHS‐positive throat swab, GABHS‐negative swab.
4. Children versus adults
There were few studies that included children (younger than 13 years of age): only 61 cases in total for when fever was evaluated at day three. There was overlap of the RR 95% CI, so that the trend for children to not experience benefits was not significantly different to adults who did (RR 1.27; 95% CI 0.76 to 2.13 and RR 0.29; 95% CI 0.06 to 1.51, respectively) (Analysis 2.3).
2.3. Analysis.
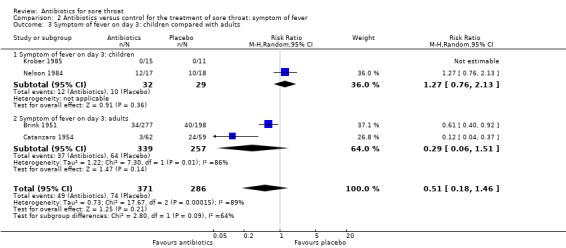
Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 3 Symptom of fever on day 3: children compared with adults.
Some of these results are summarised in Figure 3.
3.
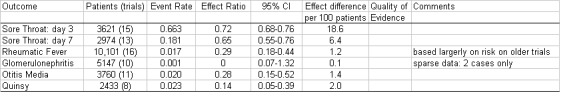
Summary of findings.
A trial from Thailand was included in the 2003 update (Leelarasamee 2000). It is especially important because it is one of the few trials from a non‐Western industrial country. Unfortunately we were unable to enter its data into the meta‐analysis because of different ways of collecting the data (in particular no data were collected mid‐way through the illness). Nevertheless, the use of antibiotics conferred no benefit (nor harms) on symptoms or complications.
Discussion
Natural history
In the placebo groups, after three days symptoms of sore throat and fever had disappeared in about 40% and 85%, respectively. Eighty‐two percent of participants were symptom‐free by one week. This natural history was similar in Streptococcus‐positive, negative and untested participants. About 1.7 per 100 placebo participants developed rheumatic fever. However, this complication occurred only in trials reporting before 1961. The background incidence of acute rheumatic fever has continued to decline in Western societies since then.
Benefits of treatment
The absolute benefit of antibiotics for the duration of symptoms was modest. The reduction of illness time is greatest in the middle of the illness period when the mean absolute reduction is about one day at around day three. There are not enough data to draw conclusions about children. The absolute reduction averaged over the whole illness can only be estimated from these data. The difference in the area under the survival curves of sore throat symptoms for those treated with placebo as opposed to antibiotic is about 16 hours for the first week.
Estimates of the number of people with sore throat who must be treated to resolve the symptoms of one by day three (the number needed to treat to benefit (NNTB)) is about 3.7 for those with positive throat swabs for Streptococcus. It is 6.5 for those with a negative swab and 14.4 for those in whom no swab has been taken. The last result is difficult to understand. Intuitively one would expect the NNTB value to lie between both the swab‐negative and swab‐positive results. Perhaps participants with less severe throat infections were recruited into the three studies in which swabs were not taken.
Antibiotics are effective at reducing the relative complication rate of people suffering sore throat. However, the relative benefit exaggerates the absolute benefit because complication rates are low and the illness is short‐lived. Interpretation of these data is aided by estimating the absolute benefit, which we attempt below.
In these trials, conducted mostly in the 1950s, for every 100 participants treated with antibiotics rather than placebo, there was one fewer case of acute rheumatic fever, two fewer cases of acute otitis media and three fewer cases of quinsy. These figures need to be adapted to current circumstances and individuals. For example, the complication rate of acute otitis media among those with sore throats before 1975 was 3%. A NNTB of about 50 to prevent one case of acute otitis media can be estimated from the data. After 1975, this complication rate fell to 0.7% and applying the odds of reducing the complication with antibiotics from the data table yields a NNTB of nearly 200 to prevent one case of acute otitis media. Clinicians will have to exercise judgement in applying these data to their patients.
In particular, in high‐income countries (where absolute rates of complications are lower) the NNTB will rise above a rate at which it might be regarded as worthwhile to treat. In low‐income countries where the absolute rate may be much higher, the lower NNTB will mean antibiotics are more likely to be effective.
Adverse effects of treatment
We were unable to present the adverse effects of antibiotic use because of inconsistencies in recording these symptoms. In other studies these were principally diarrhea, rashes and thrush (Venekamp 2013). Consideration of the side effects of antibiotics would have been useful in further defining their risk‐benefits.
Special risk groups
Acute rheumatic fever is common among people living in some parts of the world (Australian Aborigines living in low socio‐economic conditions, for example) and antibiotics may be justified to reduce the complication of acute rheumatic fever in these settings. In other parts of the world the incidence of acute rheumatic fever is so low (one estimate is that it took 12 General Practitioners' working lifetimes to encounter one new case of acute rheumatic fever in Western Scotland in the 1980s (Howie 1985)) that the risks of serious complications arising from using antibiotics for sore throat might be of the same order as that of acute rheumatic fever.
Summary of main results
1. Symptoms
Throat soreness and fever were reduced by about half when using antibiotics. The greatest difference was seen at day three. The number needed to treat to benefit (NNTB) to prevent one sore throat at day three was less than six; at week one it was 21. Antibiotics were more effective against symptoms at day three and one week if throat swabs were positive for Streptococcus compared to negative throat swabs.
2. Non‐suppurative complications
Antibiotics showed a trend for protecting against acute glomerulonephritis but there were too few cases for the results to reach statistical significance. Antibiotics reduced acute rheumatic fever by more than two‐thirds.
3. Suppurative complications
Antibiotics significantly reduced the incidence of acute otitis media by two‐thirds, acute sinusitis by a half and quinsy by 85% compared to those taking placebo.
Authors' conclusions
Antibiotics confer relative benefits in the treatment of sore throat. However, the absolute benefits are modest. Protecting sore throat sufferers against suppurative and non‐suppurative complications in high‐income countries requires treating many with antibiotics for one to benefit. This NNTB may be lower in low‐income countries. Antibiotics shorten the duration of symptoms by about 16 hours overall.
Overall completeness and applicability of evidence
The majority of trials included in this review were conducted prior to 1975, with only three trials published since 2000. The main reason for this is that very few antibiotic trials conducted recently include a placebo control arm. It is therefore unknown whether changes in bacterial resistance and population immunity over time may have altered the applicability of results.
Quality of the evidence
The quality of the evidence is considered to be moderate to high. The greatest compromise to evidence quality arose from non‐clarity in treatment allocation procedures.
Potential biases in the review process
Non‐reporting of anti‐pyretic use in a high number of studies may have constituted a source of bias in the results. Publication bias may also be considered a potential threat to the validity of results, particularly for the earlier studies.
Agreements and disagreements with other studies or reviews
A recent review analysing the risk‐benefit profile of antimicrobial prescribing for children concluded that antibiotics show little benefit in preventing quinsy following sore throat (Keith 2010). A clinical evidence review of antibiotic treatment for streptococcal pharyngitis concluded that among patients with signs and symptoms of positive bacterial infection, a specific diagnosis should be determined by performing either a throat culture or rapid antigen‐detection test, especially in children (Wessels 2011). Antibiotic treatment with penicillin or a first‐generation cephalosporin is then recommended in the case of positive bacteriologic assessment.
Authors' conclusions
Implications for practice.
Antibiotics have a beneficial effect on both suppurative and symptom reduction.
The effect on symptoms is small, so that clinicians must judge with individual cases whether it is clinically justifiable to employ antibiotics to produce this effect. In other words their use should be discretionary rather than either prohibited or mandatory. Since 90% of patients are symptom‐free by one week (whether or not treated with antibiotics), the absolute benefit of antibiotics at this time and beyond is vanishingly small.
Acute rheumatic fever is common among people living in some parts of the world (Australian Aborigines living in low socio‐economic conditions, for example) and antibiotics may be justified to reduce the incidence of this complication in these settings. For other settings where rheumatic fever is rare, there is a balance to be made between modest symptom reduction and the hazards of antimicrobial resistance.
Implications for research.
More trials are needed in low‐income countries, in socio‐economically deprived sections of high‐income countries and also in children. In high‐income countries, better prognostic studies are called for which can predict which patients may develop suppurative and non‐suppurative complications. This will help to further define which patients benefit from antibiotics.
Studies which use patient‐centred outcome measures compatible with those presented here would be greatly beneficial, in terms of easier comparison and analysis of results and ready inclusion into future updates of this review.
Few trials have attempted to measure the severity of symptoms. If antibiotics reduce the severity as well as the duration of symptoms, their benefit will have been underestimated in this meta‐analysis.
Feedback
Antibiotics for sore throat
Summary
1. The objectives as they are stated in the abstract include an assessment of the harms associated with the use of antibiotics in the management of sore throat, but the objectives as stated in the text of the review no longer refer to any assessment of harm. Indeed, the review does not address any adverse effects of antibiotics [which are not unimportant] and does not provide a reasonable explanation as to why this is not done other than to state in the discussion that this was not possible because of inconsistencies in the way these data were recorded. In the absence of RCT data on harmful effects the authors might have considered whether usable information could be provided by other study designs.
2. Reviews on this subject should treat adults and children separately, but this review does not attempt to do this.
3. All clinically important outcomes have not been addressed by the review and others such as resource use, re‐attendance and time off school or work are probably at least as important as those that were selected. It may have been more helpful to have collected data on all available outcomes provided that they are free from detection bias.
4. The question addressed by the review is not sufficiently well defined to allow the review to be executed systematically. Clear definitions are not given for the key elements of the question.
Most importantly, clear definitions of what is meant by primary care and sore throat are not given, leading to confusion around inclusion and exclusion decisions. Many of the control groups of the included studies do not involve a placebo but instead simply compare treatment with antibiotics to no treatment, so that some excluded studies would be eligible for inclusion, such as Catanzaro 1958 which was excluded because it compared antibiotics with sulfadiazine.
Apparent errors in inclusion and exclusion decisions have arisen probably as a result of the general lack of clarity discussed above. Specifically, the lack of a clear definition of what is meant by primary care appears to have led to the inclusion of an odd assortment of studies. For example, a couple of the included trials studied only people with sore throat who were admitted to hospital (Siegal 1961 and Bennike 1951). In addition, there appears to be an issue around the definition of a sore throat particularly in relation to positive or negative Streptococcus throat swabs. Streptococcal sore throats are a small sub‐set of the total population of sore throats and the failure of the reviewers to address this in the inclusion criteria means that the results of pragmatic trials of sore throat are mixed in with those of streptococcal sore throat.
There is a failure to always faithfully report the detailed results of the included studies, and there are several numerical errors in the data abstracted. For example, in Bennike 1951 the baseline numbers include patients in the "ulcerative tonsillitis" group even though most outcomes are not reported for this group.
5. The search strategy is restricted to a Medline search, a search of the Cochrane Library and citation checking. No attempt appears to have been made to search other databases. The reviewers are not explicit about the details of their searching activities nor about how they used the work of the Cochrane Acute Respiratory Infections Group.
6. References to the included and excluded studies were incomplete. Specifically they were not provided for Dagnelie 1996, Howie 1997, Little 1997 and Peterson 1997 (included) and Herx 1988, Howie 1970, Marlow 1989, McDonald 1985, Schalen 1993 and Todd 1984 (excluded).
7. Given the nature of the data presented, it is possible that a formal meta‐analysis was inappropriate. A descriptive analysis may have been more appropriate and more informative.
8. There is considerable uncertainty around the effectiveness of antibiotics on sore throat on the basis of the existing research examined by this review and this is not emphasised by the authors. Particular problems exist around the relevance of the trials to the present day with regard to the outcomes examined (rheumatic fever and glomerulonephritis), the poor quality of the majority of the included trials and the generalisability of the trials with regard to the study populations (e.g. United States air force recruits).
Jackie Young (on behalf of an interdepartmental critical appraisal workshop based in the Department of Public Health and Epidemiology, The University of Birmingham, UK) Email: j.m.young.20@bham.ac.uk
Reply
1. This is valid criticism: we need to describe the inadequacies of the information in the trials (after checking again) in the text.
2. A subgroup analysis on the basis of age is a good idea, and we will attempt this at the next major review.
3. This is a good idea, and we will attempt this at the next major review.
4. Certainly the issue of definitions is particularly difficult in this group of illnesses. One of us has written a paper on these difficulties (Del Mar C. Managing sore throat: a literature review. I. Making the diagnosis. Med J Aust 1992;156:572‐5.). There is a particular difficulty in the fact that primary care doctors use the terms 'sore throat' tonsillitis and pharyngitis in slightly different ways, including interchangeably. Moreover the notion that patients with positive swabs for Streptococcus have a different illness can be challenged. Nevertheless a subgroup analysis for this with swab‐positive and swab‐negative is a good idea which we will incorporate with our next review.
Thank for pointing numerical errors out to us, and we will check on this. Please could you detail other numerical errors for us?
5. We are explicit about our search method. At the time we undertook the search the Cochrane Acute Respiratory Infections Group had no material to assist us. This will be reviewed at the next major update.
6. Thank you for drawing our attention to this.
7. As is often the case, there is considerable variation in the population groups, treatments, outcomes measures, etc in these trials. This does not make a synthesis inappropriate, but rather allows us to examine whether these factors appear to make a difference. We also felt it important to specifically attempt to calculate the SIZE of the benefits, as this is what clinicians are interested in, and what will persuade them to modify their practice. It is then important to recognise that the size of the effect will vary in different populations: as we point out, in groups at high risk of rheumatic fever ‐ such as Australian aboriginals ‐ the prevention of RF is important; we are also interested in trying to better predict which sub‐groups will experience the most or least symptom relief, and plan to detail this in the next update.
8. We think we have discussed this in the Review. However we will reconsider what we have written in the overhaul.
Contributors
The review team.
Antibiotics for sore throat
Summary
I noticed that trials with no events in either groups are not (cannot) be part of the pooled estimates. Although I see there is a statistical/technical problem here it does not seem right. It appears to imply that no events is no evidence. I wonder whether it is defensible to add one event in both groups and add the evidence as one would normally do?
Gerben ter Riet
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
Many thanks for this. We have gone back and checked with statisticians about your point. The issue seems to be:
1. Whether empty cells are a problem. The concern is that because one cannot divide anything by zero, this might represent a problem. We think not, because in no forest plots are there totals with zero‐‐except for acute glomerulonephritis (there were no cases in the intervention arms of any trials, and only two in the control arms).
2. Whether the empty cells represent no evidence or evidence of no effect. We only recoded a zero where the study declared the outcome. Thus we assume that "no events" implies no events, rather than no reporting of events that might have occurred.
We have reported in Peto Odds ratios, the best measure for rare events.
Contributors
Chris Del Mar
Typographical error in the Abstract, 26 August 2008
Summary
Feedback: There seems to be a printing error in the abstract: the total number of cases according to the full text is 12835, but the number given in the abstract is 2835.
Martti Teikari (Feedback comment submitted 27 August 2008)
Reply
Many thanks. We will correct the typing error.
Contributors
Chris Del Mar
Antibiotics for sore throat, 30 December 2013
Summary
Comment: This work is important and useful. I have 2 concerns. First is a value judgment about the size of the treatment effect, especially concerning quinsy. Second, is the exclusion of other causes of adolescent and young adult pharyngitis ‐ group C (see Zwart 2000) strep and Fusobacterium necrophorum. Adolescents and young adults have a significant risk of suppurative complications, and most are not due to group A strep. A complete review in 2014 should acknowledge that sore throat in those age groups include other bacterial causes.
I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Robert Centor Professor Internal Medicine University of Alabama at Birmingham
Reply
We thank Dr Centor for commenting on the review with his thoughtful points.
1. Our comment on the size of the reduction of the complication of quinsy
The comments we made in the review are these:
“Antibiotics are effective at reducing the relative complication rate of people suffering sore throat. However, the relative benefit exaggerates the absolute benefit because complication rates are low and the illness is short‐lived. Interpretation of these data is aided by estimating the absolute benefit, which we attempt below.
In these trials, conducted mostly in the 1950s, for every 100 participants treated with antibiotics rather than placebo, there was one fewer case of acute rheumatic fever, two fewer cases of acute otitis media and three fewer cases of quinsy. These figures need to be adapted to current circumstances and individuals. For example, the complication rate of acute otitis media among those with sore throats before 1975 was 3%. A NNTB of about 50 to prevent one case of acute otitis media can be estimated from the data. After 1975, this complication rate fell to 0.7% and applying the odds of reducing the complication with antibiotics from the data table yields a NNTB of nearly 200 to prevent one case of acute otitis media. Clinicians will have to exercise judgement in applying these data to their patients….”
In other words we think that it is important to keep in mind the incidence of complications (and the absolute risk reduction we can expect from antibiotics) rather than simply focus on the relative risk reduction. In clinical settings (such as low‐income countries, and in Australia for example among indigenous communities) where complications are much more common, then clinicians will interpret the finding of this review by increasing the threshold for using antibiotics.
We also, incidentally, mention under “Agreements and disagreements with other studies or reviews” that “A recent review analysing the risk‐benefit profile of antimicrobial prescribing for children concluded that antibiotics show little benefit in preventing quinsy following sore throat (Keith 2010).”
2. Exclusion of the other aetiological agents of sore throat such as Group C Streptococcus and Fusobacterium necrophorum.
It is certainly true that there are many aetiological agents other than Group A beta haemolytic Streptococcus (GABHS), including a huge range of viruses and bacteria, and even non‐infective causes. However two factors influence the review:
a) The enormous focus on acute rheumatic fever as a complication, which for decades was the over‐riding indication, and the single reason proposed by researchers and clinicians for using penicillin for sore throat. This was the motivation for an enormous search to find the best way of identifying GABHS, (and incidentally the reason why your own work on predictors of GABHS was so important).
b) The availability of randomised controlled trials that addressed these agents.
In future updates, any new RCTs that address other aetiological agents will be eligible for inclusion, as can be seen from our inclusion and exclusion criteria.
Contributors
Anneliese Spinks (Feedback reply submitted 24 January 2014)
Antibiotics for sore throat, 26 September 2016
Summary
Thank you for your informative review. A previous review on, generally, the same topic was conducted by Robertson et al. (1) which included n = 10 trials. Would you comment on why the following two citations included in Robertson et al. do not appear either as included or excluded references in your review?
‐ Brock LL, Siegel AC. Studies on the prevention of rheumatic fever: the effect of time of initiation of treatment of streptococcal infections on the immune response of the host. J Clin Invest 1953, 32:630‐632. ‐ Houser HB, Eckhardt GC, Hahn EO, Denny FW, Wannamaker LW, Rammelkamp CH: Effect of aureomycin treatment of streptococcal sore throat on the streptococcal carrier state, the immunologic response of the host, and the incidence of acute rheumatic fever. Pediatrics 1953, 12(6):593‐606.
Thanks, Marlys LeBras BSP, ACPR, PharmD
References:
1. Robertson KA, Volmink JA, Mayosi BM. Antibiotics for the primary prevention of acute rheumatic fever: a meta‐analysis. BMC Cardiovascular Disorders 2005;5:11.
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Reply
We would like to thank you for alerting us to the omission of these early research studies in our review. We will seek to redress this in our coming update by reviewing the studies against our inclusion / exclusion criteria and revising the results accordingly if these studies do meet the inclusion criteria.
Contributors
Anneliese Spinks
Antibiotics for sore throat, 4 November 2019
Summary
I had a hard time understanding the assumed and corresponding risks in the Summary of Findings table ‐ the groups seem mixed up. After doing a manual calculation and also checking earlier versions I'm now convinced that you confused "placebo" and "antibiotics".
Best regards, Jon Pallon
Reply
Thank you for drawing to our attention the error in the column headings. We have now corrected this mistake so that the information in the table is consistent with the review findings.
Contributors
Anneliese Spinks
What's new
| Date | Event | Description |
|---|---|---|
| 6 January 2020 | Amended | Response to feedback comment added |
| 6 November 2019 | Feedback has been incorporated | Feedback comment added |
History
Protocol first published: Issue 1, 1997 Review first published: Issue 2, 1997
| Date | Event | Description |
|---|---|---|
| 6 October 2016 | Feedback has been incorporated | Feedback added. |
| 28 January 2014 | Feedback has been incorporated | Feedback comment and author reply added to the review. |
| 11 July 2013 | New search has been performed | Searches conducted. We did not identify any new trials for inclusion but we excluded three new trials (Kapur 2011; Kolobukhina 2011; Supajatura 2012). |
| 11 July 2013 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 18 May 2011 | New search has been performed | Searches conducted. No new studies were identified and our conclusions remain unchanged. |
| 17 February 2010 | Amended | Contact details updated. |
| 21 January 2010 | Amended | Contact details updated. |
| 25 November 2008 | New search has been performed | Searches conducted. No new studies were identified and conclusions remain unchanged. |
| 27 August 2008 | Feedback has been incorporated | Typographical error in the Abstract corrected. |
| 12 July 2008 | Amended | Converted to new review format. |
| 18 October 2006 | Feedback has been incorporated | Feedback added. |
| 9 March 2006 | New search has been performed | In this 2006 update there is an addition of data from one new study by Zwart 2003. Additionally, reported statistics were changed from odds ratios to more clinically meaningful relative risks (using a random‐effects model). Since the update for this review was submitted to The Cochrane Library (Issue 4, 2006), we have been alerted to an error in the data extraction. This error involved switching the number of participants experiencing headache on day three between the intervention and placebo groups for the study by El‐Daher 1991. We therefore incorrectly concluded that antibiotics conferred no benefit for the symptom of headache, whereas in fact the meta‐analysis does show a significant protective effect (RR 0.47; 95% CI 0.38 to 0.58). |
| 22 May 2003 | New search has been performed | Searches conducted. |
| 8 May 2000 | New search has been performed | Searches conducted. |
| 30 June 1999 | New search has been performed | Searches conducted. |
| 31 March 1996 | New search has been performed | Searches conducted. |
Acknowledgements
A previous update was completed with the help of a Glaxo sponsored educational support grant from the Australasian Cochrane Centre. The 2006 update was supported by a grant from the UK NHS through the Acute Respiratory Infections Group, based in Australia (at Bond University).
Thanks to Prof Jim Dickinson for helpful suggestions about dividing the studies into early and late last century to examine the idea that the pathogenesis of this illness, and/or its sequelae, have changed with time. Thanks to Ian Thomas and Michael Thomas for research assistance. Thanks to Beth Clewer and Katie Farmer who in January 1999 drew our attention to mistakes in the data extraction by their careful checking of original studies as part of their medical student project at the University of Bristol Medical School. The authors wish to thank the following people for commenting on the 2006 draft of this updated review: Craig Mellis, Mark Jones and Tom Fahey.
Appendices
Appendix 1. Details of previous searches
For the 2011 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 2, part of The Cochrane Library, www.thecochranelibrary.com (accessed 18 May 2011), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (November 2008 to May week 1, 2011) and EMBASE (November 2008 to May 2011).
In the previous update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2008, Issue 4) which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to November 2008) and EMBASE (January 1990 to November 2008).
MEDLINE and CENTRAL were searched using the search strategy shown below. We combined the MEDLINE search string with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity and precision‐maximising version (2008 revision) (Lefebvre 2011). We adapted the search string for EMBASE.
MEDLINE (Ovid)
# 1 explode Pharyngitis/ # 2 pharyngit$.mp. # 3 explode Nasopharyngitis/ # 4 nasopharyngit$.mp. # 5 explode Tonsillitis/ # 6 tonsillit$.mp. # 7 sore throat*.mp. # 8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 # 9 explode Anti‐Bacterial Agents/ # 10 antibiot$.mp. # 11 #9 OR #10 # 12 #8 AND #11
(Embase.com used in 2011 update) #1. 'pharyngitis'/exp AND [embase]/lim #2. pharyngit*:ti,ab AND [2004‐2008]/py #3. 'rhinopharyngitis'/exp AND [embase]/lim #4. rhinopharyngit*:ti,ab OR nasopharyngit*:ti,ab [embase]/lim #5. 'tonsillitis'/exp AND [embase]/lim #6. tonsillit*:ti,ab AND [embase]/lim #7. 'sore throat'/exp AND [embase]/lim #8. 'sore throat':ti,ab OR 'sore throats':ti,ab embase]/lim #9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10. 'antibiotic agent'/exp AND [embase]/lim #11. antibiotic*:ti,ab AND [embase]/lim #12. #10 OR #11 619,306 #13. random*:ti,ab OR factorial*:ti,ab OR crossover*:ti,ab OR 'cross over':ti,ab OR placebo*:ti,ab OR assign*:ti,ab OR allocat*:ti,ab OR volunteer*:ti,ab AND [embase]/lim #14. 'double blind':ti,ab OR 'double blinded':ti,ab OR 'single blind':ti,ab OR 'single blinded':ti,ab AND [embase]/lim #15. 'crossover procedure'/exp AND [embase]/lim #16. 'double blind procedure'/exp AND [embase]/lim #17. 'single blind procedure'/exp AND [embase]/lim #18. 'randomized controlled trial'/exp AND [embase]/lim #19. #13 OR #14 OR #15 OR #16 OR #17 OR #18 #20. #9 AND #12 AND #19
(EMBASE search used in earlier versions of the review) EMBASE (WebSPIRS) #1 explode 'pharyngitis‐' / all subheadings in DEM,DER,DRM,DRR #2 (pharyngit* in ti) or (pharyngit* in ab) #3 explode 'rhinopharyngitis‐' / all subheadings in DEM,DER,DRM,DRR #4 (nasopharyngit* in ti) or (nasopharyngit* in ab) #5 explode 'tonsillitis‐' / all subheadings in DEM,DER,DRM,DRR #6 (tonsillit* in ti) or (tonsillit* in ab) #7 explode 'sore‐throat' / all subheadings in DEM,DER,DRM,DRR #8 (sore throat in ti) or (sore throat in ab) #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 'antibiotic‐agent' / all subheadings in DEM,DER,DRM,DRR #11 (antibiotic* in ti) or (antibiotic* in ab) #12 #10 or #11 #13 #9 and #12 #14 explode 'randomized‐controlled‐trial' / all subheadings #15 explode 'controlled‐study' / all subheadings #16 explode 'single‐blind‐procedure' / all subheadings #17 explode 'double‐blind‐procedure' / all subheadings #18 explode 'crossover‐procedure' / all subheadings #19 explode 'phase‐3‐clinical‐trial' / all subheadings #20 (randomi?ed controlled trial in ti) or (randomi?ed controlled trial in ab) #21 ((random* or placebo* or double‐blind*)in ti) or ((random* or placebo* or double‐blind*)in ab) #22 (controlled clinical trial* in ti) or (controlled clinical trial* in ab) #23 (explode 'randomized‐controlled‐trial' / all subheadings) or (explode 'controlled‐study' / all subheadings) or (explode 'single‐blind‐procedure' / all subheadings) or (explode 'double‐blind‐procedure' / all subheadings) or (explode 'crossover‐procedure' / all subheadings) or (explode 'phase‐3‐clinical‐trial' / all subheadings) or ((randomi?ed controlled trial in ti) or (randomi?ed controlled trial in ab)) or (((random* or placebo* or double‐blind*)in ti) or ((random* or placebo* or double‐blind*)in ab)) or ((controlled clinical trial* in ti) or (controlled clinical trial* in ab)) #24 (nonhuman in der) not ((human in der)and (nonhuman in der)) #25 ((explode 'randomized‐controlled‐trial' / all subheadings) or (explode 'controlled‐study' / all subheadings) or (explode 'single‐blind‐procedure' / all subheadings) or (explode 'double‐blind‐procedure' / all subheadings) or (explode 'crossover‐procedure' / all subheadings) or (explode 'phase‐3‐clinical‐trial' / all subheadings) or ((randomi?ed controlled trial in ti) or (randomi?ed controlled trial in ab)) or (((random* or placebo* or double‐blind*)in ti) or ((random* or placebo* or double‐blind*)in ab)) or ((controlled clinical trial* in ti) or (controlled clinical trial* in ab))) not ((nonhuman in der) not ((human in der)and (nonhuman in der))) #26 #13 and #25
Appendix 2. EMBASE (Elsevier) search strategy
#16 #11 AND #15 #15 #12 OR #13 OR #14 #14 azithromycin*:ab,ti OR clarithromycin*:ab,ti OR erythromycin*:ab,ti OR roxithromycin*:ab,ti OR macrolide*:ab,ti OR cefamandole*:ab,ti OR cefoperazone*:ab,ti OR cefazolin*:ab,ti OR cefonicid*:ab,ti OR cefsulodin*:ab,ti OR cephacetrile*:ab,ti OR cefotaxime*:ab,ti OR cephalothin*:ab,ti OR cephapirin*:ab,ti OR cephalexin*:ab,ti OR cephaclor*:ab,ti OR cephadroxil*:ab,ti OR cephaloglycin*:ab,ti OR cephradine*:ab,ti OR cephaloridine*:ab,ti OR ceftazidime*:ab,ti OR cephamycin*:ab,ti OR cefmetazole*:ab,ti OR cefotetan*:ab,ti OR cefoxitin*:ab,ti OR cephalosporin*:ab,ti OR cefpodoxime*:ab,ti OR cefuroxime*:ab,ti OR cefixime*:ab,ti OR amoxicillin*:ab,ti OR amoxycillin*:ab,ti OR ampicillin*:ab,ti OR sulbactum*:ab,ti OR tetracyclin*:ab,ti OR clindamycin*:ab,ti OR lincomycin*:ab,ti OR doxycyclin*:ab,ti OR fluoroquinolone*:ab,ti OR ciprofloxacin*:ab,ti OR fleroxacin*:ab,ti OR enoxacin*:ab,ti OR norfloxacin*:ab,ti OR ofloxacin*:ab,ti OR pefloxacin*:ab,ti OR moxifloxacin*:ab,ti OR esparfloxacin*:ab,ti OR clindamicin*:ab,ti OR penicillin*:ab,ti OR ticarcillin*:ab,ti OR 'beta‐lactam':ab,ti OR 'beta‐lactams':ab,ti OR levofloxacin*:ab,ti OR trimethoprim*:ab,ti OR 'co‐trimoxazole':ab,ti #13 antibiot*:ab,ti #12 'antibiotic agent'/exp #11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #10 (sore* NEAR/2 throat*):ab,ti #9 ((throat* OR pharyn*) NEAR/3 (infect* OR inflam* OR strep*)):ab,ti #8 'sore throat'/de #7 (tonsil* NEAR/2 (infect* OR inflam*)):ab,ti #6 tonsillit*:ab,ti #5 'tonsillitis'/exp #4 rhinopharyngit*:ab,ti OR nasopharyngit*:ab,ti #3 'rhinopharyngitis'/de #2 pharyngit*:ab,ti #1 'pharyngitis'/exp
Data and analyses
Comparison 1. Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom of sore throat on day 3 | 15 | 3621 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.79] |
| 2 Symptom of sore throat on day 3: blind versus unblinded studies | 15 | 3621 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.79] |
| 2.1 Symptom of sore throat on day 3: blinded studies | 12 | 2662 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.54, 0.78] |
| 2.2 Symptom of sore throat on day 3: unblinded studies | 3 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.05] |
| 3 Symptom of sore throat on day 3: antipyretics versus no antipyretics | 5 | 1137 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.70] |
| 3.1 Symptom of sore throat on day 3: antipyretics administered | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.33, 0.81] |
| 3.2 Symptom of sore throat on day 3: no antipyretics administered | 2 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.55, 0.70] |
| 4 Symptom of sore throat on day 3: GABHS‐positive throat swab, negative swab, untested/inseparable | 15 | 3600 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.78] |
| 4.1 Symptom of sore throat on day 3: GABHS‐positive throat swab | 11 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.71] |
| 4.2 Symptom of sore throat on day 3: GABHS‐negative throat swab | 6 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| 4.3 Symptom of sore throat on day 3: untested for GABHS culture or combined inseparable data | 3 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 5 Symptom of sore throat at one week (6 to 8 days) | 13 | 2974 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.76] |
| 6 Symptom of sore throat at one week (6 to 8 days): blind versus unblinded studies | 13 | 2944 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| 6.1 Symptom of sore throat at 1 week (6 to 8 days): blinded studies | 9 | 1616 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.38, 1.03] |
| 6.2 Symptom of sore throat at 1 week (6 to 8 days): unblinded studies | 4 | 1328 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.08, 1.15] |
| 7 Symptom of sore throat at one week (6 to 8 days): GABHS‐positive throat swab, GABHS‐negative swab | 12 | 2524 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.80] |
| 7.1 Symptom of sore throat at 1 week (6 to 8 days): GABHS‐positive throat swab | 7 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 7.2 Symptom of sore throat at 1 week (6 to 8 days): GABHS‐negative throat swab | 5 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.07] |
| 7.3 Symptom of sore throat at 1 week (6 to 8 days): GABHS untested | 3 | 866 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.03, 4.47] |
Comparison 2. Antibiotics versus control for the treatment of sore throat: symptom of fever.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom of fever on day 3 | 7 | 1334 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.10] |
| 2 Symptom of fever on day 3: blinded versus unblinded studies | 7 | 1334 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.10] |
| 2.1 Symptom of fever on day 3: blinded studies. | 4 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.54, 1.23] |
| 2.2 Symptom of fever on day 3: unblinded studies. | 3 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.31, 1.37] |
| 3 Symptom of fever on day 3: children compared with adults | 4 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.46] |
| 3.1 Symptom of fever on day 3: children | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.76, 2.13] |
| 3.2 Symptom of fever on day 3: adults | 2 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.51] |
| 4 Symptom of fever at 1 week (6 to 8 days) | 3 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.2. Analysis.
Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 2 Symptom of fever on day 3: blinded versus unblinded studies.
2.4. Analysis.
Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 4 Symptom of fever at 1 week (6 to 8 days).
Comparison 3. Antibiotics versus control for the treatment of sore throat: symptom of headache.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom of headache on day 3 | 3 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.71] |
| 2 Symptom of headache on day 3: blinded versus unblinded studies | 3 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.71] |
| 2.1 Symptom headache on day 3: blinded studies | 2 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.20] |
| 2.2 Symptom of headache on day 3: unblinded studies | 1 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.72] |
3.2. Analysis.
Comparison 3 Antibiotics versus control for the treatment of sore throat: symptom of headache, Outcome 2 Symptom of headache on day 3: blinded versus unblinded studies.
Comparison 4. Antibiotics versus placebo for the treatment of sore throat: incidence of complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of acute rheumatic fever within 2 months. Rheumatic fever defined by clinical diagnosis | 16 | 10101 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.60] |
| 2 Incidence of acute rheumatic fever within 2 months. Penicillin versus placebo | 14 | 8175 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.14, 0.50] |
| 3 Incidence of acute rheumatic fever within 2 months: early (pre‐1975) versus late studies (post‐1975) | 16 | 10101 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.60] |
| 3.1 Incidence of acute rheumatic fever within 2 months: early (pre‐1975) studies | 10 | 7617 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.60] |
| 3.2 Incidence of acute rheumatic fever within 2 months: late (post‐1975) studies | 6 | 2484 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Incidence of otitis media within 14 days. Otitis media defined by clinical diagnosis | 11 | 3760 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.58] |
| 5 Incidence of otitis media within 14 days: early (pre‐1975) versus late studies (post‐1975) | 11 | 3760 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.58] |
| 5.1 Incidence of otitis media within 14 days: early (pre‐1975) studies | 5 | 1837 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.62] |
| 5.2 Incidence of otitis media within 14 days: late (post‐1975) studies | 6 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.74] |
| 6 Incidence of sinusitis within 14 days. Sinusitis defined by clinical diagnosis | 8 | 2387 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.08, 2.76] |
| 7 Incidence of quinsy within 2 months. Quinsy defined by clinical diagnosis | 8 | 2433 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.05, 0.47] |
| 8 Incidence of acute glomerulonephritis within 1 month. Acute glomerulonephritis defined by clinical diagnosis | 10 | 5147 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 2.08] |
4.3. Analysis.

Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 3 Incidence of acute rheumatic fever within 2 months: early (pre‐1975) versus late studies (post‐1975).
4.5. Analysis.
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 5 Incidence of otitis media within 14 days: early (pre‐1975) versus late studies (post‐1975).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bennike 1951.
| Methods | Open study, quasi‐randomised | |
| Participants | 669 patients aged from less than 1 year to older than 50 years of age. Research was divided into 3 studies: ordinary tonsillitis, "phlegmonous" tonsillitis and "ulcerative" tonsillitis. Participants were excluded if they had a complication of tonsillitis on admission or if they had previous antibiotic treatment for the present sore throat | |
| Interventions | Age‐adjusted intramuscular penicillin twice daily for 6 days or no treatment as a control condition | |
| Outcomes | Incidence of rheumatic fever, otitis media, quinsy, sinusitis and symptoms of sore throat and headache | |
| Notes | No antipyretics were administered to the control group. The use of antipyretics to participants in the treatment group was unstated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants allocated to alternate conditions on alternate days |
| Allocation concealment (selection bias) | High risk | No concealment of allocation present |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | No antipyretics were administered to the control group. The use of antipyretics to participants in the treatment group was unstated |
Brink 1951.
| Methods | Open study | |
| Participants | 395 young adult males recruited into United States Air Force | |
| Interventions | Intramuscular penicillin over 4 days, chlortetracycline for 3 days or no treatment as control group | |
| Outcomes | Incidence of rheumatic fever, otitis media and symptoms of sore throat, fever and headache | |
| Notes | No antipyretics were administered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised by Air Force serial number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Brumfitt 1957.
| Methods | Open study | |
| Participants | 121 young adult men, aged 18 to 21 years, recruited into United States Air Force. Participants were excluded from study if their temperature was below 99.3 degrees F, if they had sore throat for more than 72 hours prior to presentation, or if they had some other generalised illness | |
| Interventions | Intramuscular penicillin twice‐daily for 4 days or no treatment as a control condition | |
| Outcomes | Incidence of rheumatic fever and symptoms of sore throat and fever | |
| Notes | Aspirin gargles were given 6‐hourly. Whether participants were permitted to swallow the aspirin was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised by hospital bed number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Catanzaro 1954.
| Methods | Single‐blind, participants were unaware of treatment type, placebo‐controlled trial. The outcome of treatment was not determined blind | |
| Participants | 640 young adult males recruited into United States Air Force. Missing data were not explained Data from participants who produced a GABHS‐negative throat swab were excluded. Participants were excluded if they presented with a suppurative complication at the time of admission | |
| Interventions | Intramuscular penicillin administered for 5 days, sulphonamide administered for 5 days or no treatment as a control condition | |
| Outcomes | Incidence of rheumatic fever | |
| Notes | Antipyretic use was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised by Air Force serial number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Chamovitz 1954.
| Methods | Single‐blind placebo study | |
| Participants | 366 young adult males recruited into United States Air Force. Participants were excluded if they had previously developed rheumatic fever, had previous penicillin reaction or if they had a suppurative complication at the time of admission | |
| Interventions | Intramuscular penicillin | |
| Outcomes | Incidence of rheumatic fever, otitis media and sinusitis | |
| Notes | Antipyretic use was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised by Air Force serial number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants did not know treatment type they were receiving. The outcome of treatment was not determined blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Chapple 1956.
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | 308 participants older than 2 years. Data from 283 participants included in analyses | |
| Interventions | Age‐adjusted oral penicillin, sulphadimidine or barium sulphate (placebo) administered for 5 days | |
| Outcomes | Incidence of rheumatic fever, otitis media and symptom of sore throat | |
| Notes | All groups received controlled doses of antipyretics twice daily for 3 days Data from only 200 participants presenting with sore throat on day 1 included in sore throat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised by random bottle dispensing |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Dagnelie 1996.
| Methods | Randomised, double‐blind, placebo‐controlled trial of penicillin V on the course and bacteriological response in patients with sore throat in general practice | |
| Participants | 239 patients aged 4 to 60, presenting with sore throat to 37 General Practices in the Netherlands, who were clinically suspected of GABHS | |
| Interventions | Treatment with either penicillin V or placebo | |
| Outcomes | Resolution of sore throat, fever and return to daily activities (assessed by doctor and by diary for 7 days) | |
| Notes | * Need raw data to make this study comparable to the meta‐analysis, however data are available for sore throat on day 3 and quinsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition of participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
De Meyere 1992.
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | 173 participants aged 5 to 50 years, from the Gent region of Belgium Data were obtained from 173 participants on days 1 and 3 Data were obtained from 131 participants on days 2, 4, 5, 6 and 7 Participants excluded if they: produced a GABHS‐negative throat swab, had a sore throat for greater than 5 days, had a previous history of acute rheumatic fever, had an allergy to beta‐lactam antibiotics, had received any antibiotics within the past 14 days, were in any high‐risk situation as determined by the physician | |
| Interventions | Oral penicillin or oral placebo 3 times a day | |
| Outcomes | Symptom of sore throat All data obtained, except from days 1 and 3, were self reported from a diary | |
| Notes | Antipyretics were used as required by participants. Use of antipyretics and other symptom‐relieving methods was documented in a diary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not documented |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Denny 1950.
| Methods | Single‐blind study. The outcome was determined blind on follow‐up by physicians who did not know what treatment type each participant had received | |
| Participants | 1602 young adult males recruited into United States Air Force | |
| Interventions | Intramuscular penicillin for 4 days or no treatment as a control group | |
| Outcomes | Incidence of rheumatic fever only | |
| Notes | Antipyretic use was not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised by Air Force serial number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single blind study ‐ assessment was conducted by physicians who were unaware of treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not stated |
Denny 1953.
| Methods | Single‐blind, randomised, placebo‐controlled trial. Outcome determined blind by physicians who did not know treatment type | |
| Participants | 103 young adult males recruited in the United States Air Force. Participants were excluded if they had no exudate on their tonsils or larynx, if they had a leukocyte count of less than 10,000; or if they had experienced symptoms of sore throat for more than 31 hours | |
| Interventions | Intramuscular penicillin daily for 5 days, oral aureomycin or oral terramycin administered every 6 hours for 3 days or oral lactose placebo for 3 days as a control condition | |
| Outcomes | Incidence of acute rheumatic fever, otitis media, quinsy, sinusitis and symptoms of sore throat and headache | |
| Notes | No antipyretics were administered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to treatment groups by drawing a card from a deck |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blind study ‐ assessment was conducted by physicians who were unaware of treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
El‐Daher 1991.
| Methods | Double‐blinded, randomised controlled trial | |
| Participants | 229 children with positive culture for GABHS | |
| Interventions | Early treatment with oral penicillin for 10 days versus oral placebo for 2 days followed by oral penicillin for 8 days | |
| Outcomes | Symptoms of sore throat and headache on day 3 | |
| Notes | Examination of participants was done on day 3 before administering penicillin to placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition of participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Howe 1997.
| Methods | 22 GPs in one region of the UK recruited | |
| Participants | 154 patients aged 16 to 60 years presenting to their GP with sore throat and for whom the GP would normally prescribe an antibiotic | |
| Interventions | Therapy with either penicillin V (250 mg 4 times a day), cefixime (200 mg daily) or placebo | |
| Outcomes | Resolution of a composite "symptom score" with time; eradication of GABHS. A diary was kept of symptom resolution over 7 days | |
| Notes | *Symptom results were bundled into a composite "symptom score". The raw data on sore throat, cough and fever resolution has been requested from the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation scheme (performed in blocks of 6) |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Krober 1985.
| Methods | Double‐blind placebo trial | |
| Participants | 44 children presenting to a paediatric clinic. 26 of these participants yielded GABHS‐positive throat swabs Participants were excluded if: the duration of symptoms was greater than 72 hours; they had received oral antibiotics within the past 72 hours or intramuscular antibiotics within the past 30 days; they had history of penicillin allergy; they had a rash suggestive of scarlet fever; they had a concurrent infection that required antibiotics other than penicillin; or if they had severe illness requiring immediate penicillin treatment Participants who produced GABHS‐negative throat swabs were excluded from the study | |
| Interventions | Oral penicillin or similar looking and tasting oral placebo for the control condition, 3 times a day for 3 days | |
| Outcomes | Symptom of fever | |
| Notes | Antipyretic use was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by table of random numbers |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Landsman 1951.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 95 participants who presented to general practice complaining of sore throat | |
| Interventions | Oral sulphonamide or similar looking and tasting oral placebo, for the control condition | |
| Outcomes | Incidence of sinusitis or quinsy or symptoms of sore throat or fever | |
| Notes | Antipyretic use was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by random numbering of bottles |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Leelarasamee 2000.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 1217 patients aged over 5 years presenting to 4 community‐based medical centres with complaints of fever or sore throat of less than 10 days duration | |
| Interventions | Participants were randomised to receive either amoxycillin or placebo for 7 days | |
| Outcomes | Duration of sore throat and fever. Incidence of complications and adverse reactions | |
| Notes | Antipyretics were given if deemed necessary by physicians | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some loss to follow‐up occurred |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Little 1997.
| Methods | Unblinded randomised trial | |
| Participants | 716 patients aged 4 years and over, presenting to their GP with a sore throat, with an abnormal physical finding localised to the throat (e.g. inflamed tonsils or pharynx, etc.) | |
| Interventions | Participants were randomised to 3 groups. Participants in the first group were given an antibiotic for 10 days; those in the second group were given no prescription; and in the third group were given an offer of antibiotic prescription if the symptoms were not starting to settle after 3 days | |
| Outcomes | Main outcomes ‐ duration of symptoms, satisfaction and compliance with and perceived efficacy of antibiotics, time off school or work. Participants given a daily diary in which to record symptoms and temperature. Participants who did not return diaries were followed up over the phone | |
| Notes | Participants randomised, but neither participants nor doctors blinded to the therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or assessors was performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition of participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
MacDonald 1951.
| Methods | Outcome determined blind | |
| Participants | 82 young adult males recruited into the United States Air Force 41 in treatment group; 41 in control group | |
| Interventions | Oral sulphatriad or identical oral lactose placebo, administered to the control condition, taken every 4 hours | |
| Outcomes | Symptom of sore throat | |
| Notes | Antipyretics were administered to 1 participant in the treatment group and 2 participants in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised by Air Force serial number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcomes were determined blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Middleton 1988.
| Methods | Multicentre, double‐blind, randomised, placebo‐controlled | |
| Participants | 178 participants aged 4 to 29 years with streptococcal pharyngitis. Participants had symptom duration of less than 4 days. Results reported for 57 participants with severe illness only | |
| Interventions | 8 individual doses of penicillin or placebo | |
| Outcomes | Symptoms of sore throat and fever | |
| Notes | Phone report after 48 hours used to measure outcome at day 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition of participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Nelson 1984.
| Methods | An oral placebo was used to single‐blind participants, however outcome was not determined blind | |
| Participants | 51 children aged 5 to 11 years. Sixteen participants were excluded because they did not produce GABHS‐positive throat swabs, leaving 35 participants. Children with history of penicillin hypersensitivity were also excluded | |
| Interventions | Intramuscular penicillin or oral syrup placebo as a control group | |
| Outcomes | Symptoms of sore throat and fever | |
| Notes | No antipyretics were administered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised to conditions by hospital number allocation |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | An oral placebo was used to single‐blind participants. However outcome was not determined blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Petersen 1997.
| Methods | Randomised placebo‐controlled trial of participants' culture‐negative for GABHS | |
| Participants | 186 adults (aged 18 to 50) presenting to an ambulatory setting, whose chief complaint was sore throat and whose GABHS culture was subsequently found to be negative | |
| Interventions | Treatment with either erythromycin (333 mg, 3 times daily) or placebo | |
| Outcomes | Main outcomes ‐ time to improvement in sore throat, cough, activity level and sense of well‐being. Participants completed a daily questionnaire on the progress of outcome measures. Follow‐up visits were arranged 2 to 3 weeks after enrolment to repeat cultures, collect diaries and assess compliance | |
| Notes | It is not clear how many participants kept diaries for the sore throat data in each group. Authors excluded GABHS‐positive patients (15 out of 212 initially randomised) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear how many participants kept diaries for the sore throat data in each group. Authors excluded GABHS‐positive participants (15 out of 212 initially randomised) |
Pichichero 1987.
| Methods | Single‐blind, randomised, placebo‐controlled trial | |
| Participants | 114 GABHS‐positive children aged 4 to 18 years. Children were excluded from the study if: a throat swab was negative for GABHS; were allergic to penicillin; had received penicillin in past 7 days; had another acute illness within 7 days, had a GABHS‐positive swab in past month, or had another concurrent infection that required antibiotics | |
| Interventions | Oral penicillin for 48 hours or an identical‐looking and tasting oral placebo used for the control condition | |
| Outcomes | Incidence of otitis media, quinsy or sinusitis | |
| Notes | Antipyretics administered 4‐hourly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant attrition |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Siegel 1961.
| Methods | Randomised controlled trial | |
| Participants | 1213 children aged 3 to 16 years. Suppurative complications occurring in participants in the control condition were treated with sulphonamides. Participants were excluded if they had a complication on admission | |
| Interventions | Intramuscular penicillin or no treatment for the controls | |
| Outcomes | Incidence of rheumatic fever | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised by bed chart number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Taylor 1977.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 122 children aged 2 to 10 years. Children with positive Streptococcus throat swabs were excluded 9 children were excluded during trial because of pre‐existing suppurative complications | |
| Interventions | Oral amoxycillin, oral cotrimoxazole or an oral placebo was administered by parents 3 times a day for 5 days | |
| Outcomes | Incidence of otitis media and sinusitis and symptoms of sore throat and fever | |
| Notes | Antipyretic use was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation to groups was not documented |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Wannamaker 1951.
| Methods | Single‐blind study. The intervention outcomes were determined by physicians who were unaware of participant treatment allocation | |
| Participants | 1974 young adult males recruited into the United States Air Force | |
| Interventions | Intramuscular penicillin over 1 to 3 days or no treatment for the control condition | |
| Outcomes | Incidence of rheumatic fever | |
| Notes | Antipyretic use was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised to groups by Air Force serial number |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Whitfield 1981.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | Participants were people who presented to the General Practitioner with sore throat, aged more than 10 years. 745 participants were commenced on the study. Only 528 returned questionnaires. Participants were excluded if the General Practitioner thought the participant would demonstrate poor compliance; if they had previous reaction to penicillin; or a previous episode of rheumatic fever or acute nephritis | |
| Interventions | Oral penicillin 4 times a day for 5 days or identical‐looking and tasting oral lactose placebo 4 times a day for 5 days | |
| Outcomes | Symptom of fever | |
| Notes | Antipyretic use was not documented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by pre‐determined random order |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Antipyretic use was not documented |
Zwart 2000.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 561 participants aged 15 to 60 years presenting with sore throat of less than 7 days duration | |
| Interventions | Penicillin V for 7 days, penicillin V for 3 days followed by 4 days of placebo or placebo or 7 days | |
| Outcomes | Resolution of symptoms and recurrence of sore throat | |
| Notes | Author was contacted for data that could be used in the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
Zwart 2003.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 156 children aged 4 to 15 years presenting with sore throat of less than 7 days duration with at least 2 of 4 Centor criteria | |
| Interventions | Penicillin V for 7 days, penicillin V for 3 days followed by 4 days of placebo or placebo or 7 days | |
| Outcomes | Duration of symptoms of sore throat, occurrence of streptococcal sequelae | |
| Notes | Author was contacted for data that could be used in the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
F: Farenheit GABHS: group A beta haemolytic Streptococcus GP: general practitioner
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barwitz 1999 | Participants were randomised to 2 GPs for subsequent treatment with different management protocols |
| Bass 1986 | Study used a Likert scale to measure severity and duration of symptoms. No raw scores are available for entry into meta‐analysis |
| Bishop 1952 | Non‐randomised allocation to treatment groups. (Quote) "Where an exceptionally severe case fell in the control group and it was felt unjustifiable to withhold specific treatment, the case was transferred to one of the other groups and the next case was placed in the control group." This bias was not quantified |
| Catanzaro 1958 | Study compared sulphonamides with other antibiotics. No control condition was used |
| Cruickshank 1960 | Study is another report of the data previously published by Brumfitt 1957 |
| Dowell 2001 | Cough was the main complaint for patients, not sore throat |
| Gerber 1985 | Study compared 2 different regimens of penicillin. No placebo control group was used |
| Gerber 1989 | Assessed 2 regimes of penicillin. No control group used |
| Ginsburg 1980 | Study compared penicillin V with cefadroxil. No placebo control group was used |
| Guthrie 1988 | Study did not use control condition |
| Haverkorn 1971 | Participants not treated with antibiotics given antipyretics. Participants receiving antibiotics received no antipyretics. No control condition |
| Herz 1988 | No participant‐centred outcomes, except return visits for URIs Poor randomisation ‐ out of a series of 202, the first and last 50 were assigned to antibiotics, with the middle 102 assigned to control |
| Howie 1970 | Illness was "cold or flu‐like illness", not acute pharyngitis (exclusively). Soreness of throat not an outcome measure |
| Jensen 1991 | Participants were not randomly allocated to treatment groups and were not blinded to treatment |
| Kapur 2011 | No intervention was provided to participants. Study tracked natural course of illness only |
| Kolobukhina 2011 | Study investigated the combination of Ingavirin (antiviral medication) with an antibacterial agent in adults with viral respiratory infections. No comparison of antibiotics alone against placebo |
| Marlow 1989 | Participant population highly selected (non‐pregnant, negative rapid strep. test, negative throat culture, no other infection present, not allergic to erythromycin, aged older than 12) and participant‐centred outcomes not compatible with those in this meta‐analysis |
| Massell 1951 | Study examined effect of penicillin on haemolytic streptococci infections in rheumatic patients only, without randomisation to control condition. Infections that were not treated with penicillin for 'various reasons' were treated as controls. These reasons were not given |
| McDonald 1985 | No data suitable for this meta‐analysis were described although symptoms were recorded. The author was approached for these data, but no reply was received |
| Merenstein 1974 | No data on suppurative or non‐suppurative complications No data on day 3 for soreness of throat, fever or headache |
| Morris 1956 | Study observed effect of sulfadiazine on prevention of rheumatic fever only. No control condition was used |
| Nasonova 1999 | Study a controlled clinical trial without randomisation of participants |
| Pandraud 2002 | Investigation of effect of fusafungine on chronic conditions of follicular pharyngitis. Not relevant for this review |
| Randolph 1985 | No data on suppurative or non‐suppurative complications No data on day 3 or 7 for soreness of throat, fever or headache |
| Schalen 1985 | Primary complaint hoarseness, not sore throat. No patient‐centred outcomes apart from hoarseness |
| Schalen 1993 | Patients presented for laryngitis and hoarseness, not pharyngitis |
| Schwartz 1981 | Study compared 7 versus 10 days of treatment with penicillin. No control group was used |
| Shevrygin 2000 | Study was a clinical trial without a control condition |
| Shvartzman 1993 | Study compared efficacy of amoxycillin against penicillin, no control condition was used |
| Stillerman 1986 | Study compared penicillin with cephalosporins, no control group was used |
| Stromberg 1988 | No placebo control group was used. Study compared different antibiotic regimens |
| Supajatura 2012 | Antibiotics were not offered as an intervention. Study investigated the efficacy of Mangosteen spray against placebo only |
| Todd 1984 | Primary complaint not sore throat ‐ purulent nasopharyngitis instead |
| Valkenburg 1971 | Study did not involve any control measures. Data only given for participants not treated with antibiotics |
GP: general practitioner URIs: upper respiratory infections
Contributions of authors
Chris Del Mar first conceived the review, presenting it as a meta‐analysis in a journal (Del Mar 1992a; Del Mar 1992b). It was subsequently improved and modified for The Cochrane Library with Paul Glasziou (who improved the subgroup analyses) and Anneliese Spinks (who updated searches and completed the analyses).
Sources of support
Internal sources
Bond University (2006 update), Australia.
University of Oxford, UK.
Griffith University, Australia.
External sources
NHS support, UK.
Declarations of interest
Paul Glasziou is on the board of Therapeutic Guidelines Limited and holds a research grant from the NHMRC on antibiotic resistance.
Chris Del Mar has received funding from the NHMRC for antibiotic resistance, funding the ARI Cochrane Group, and from some consultancies (GSK for advice about vaccines for otitis media; and a local pharmaceutical company contemplating analgesic ear drops for otitis media).
Anneliese Spinks does not have any interests to declare relevant to this review.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Bennike 1951 {published data only}
- Bennike TBMK, Kjaer E, Skadhauge K, Trolle E. Penicillin therapy in acute tonsillitis, phlegmonous tonsillitis and ulcerative tonsillitis. Acta Medica Scandinavica 1951;139:253‐74. [DOI] [PubMed] [Google Scholar]
Brink 1951 {published data only}
- Brink WRR, Denny FW, Wannamaker LW. Effect of penicillin and aureomycin on the natural course of streptococcal tonsillitis and pharyngitis. American Journal of Medicine 1951;10:300‐8. [DOI] [PubMed] [Google Scholar]
Brumfitt 1957 {published data only}
- Brumfitt WS, Slater DH. Treatment of acute sore throat with penicillin: a controlled trial among young soldiers. Lancet 1957;272(6958):8‐11. [DOI] [PubMed] [Google Scholar]
Catanzaro 1954 {published data only}
- Catanzaro FJ, Morris AJ, Chamovitz R, Rammelkamp CH, Stolzer B, Perry WD. The role of Streptococcus in the pathogenesis of rheumatic fever. American Journal of Medicine 1954;17(6):749‐56. [DOI] [PubMed] [Google Scholar]
Chamovitz 1954 {published data only}
- Chamovitz R, Stetson CA, Rammelkamp CH. Prevention of rheumatic fever by treatment of previous streptococcal infections. New England Journal of Medicine 1954;251(12):466‐71. [DOI] [PubMed] [Google Scholar]
Chapple 1956 {published data only}
- Chapple LM, Paulett JD, Tuckman E, Woodall JT, Tomlinson AJH, McDonald JC. Treatment of acute sore throat in general practice. British Medical Journal 1956;March(4969):705‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dagnelie 1996 {published and unpublished data}
- Dagnelie CF, van‐der‐Graaf Y, Melker RA. Do patients with sore throat benefit from penicillin? A randomised double‐blind placebo‐controlled clinical trial with penicillin V in general practice. British Journal of General Practice 1996;46(411):589‐93. [PMC free article] [PubMed] [Google Scholar]
De Meyere 1992 {published data only}
- Meyere M, Mervielde Y, Verschraegen G, Bogaert M. Effect of penicillin on the clinical course of streptococcal pharyngitis in general practice. European Journal of Clinical Pharmacology 1992;43(6):581‐5. [DOI] [PubMed] [Google Scholar]
Denny 1950 {published data only}
- Denny LW, Brink WR, Rammelkamp CH, Custer EA. Prevention of rheumatic fever: treatment of the preceding streptococcal infection. Journal of the American Medical Association 1950;143(2):151‐3. [DOI] [PubMed] [Google Scholar]
Denny 1953 {published data only}
- Denny LW, Hahn EO. Comparative effects of penicillin, aureomycin and terramycin on streptococcal tonsillitis and pharyngitis. Pediatrics 1953;11(1):7‐14. [PubMed] [Google Scholar]
El‐Daher 1991 {published data only}
- El‐Daher NT, Hijazi SS, Rawashdeh NM, Al‐Khalil IA, Abu‐Ektaish FM, Abdel‐Latif DI. Immediate vs. delayed treatment of Group A beta‐hemolytic streptococcal pharyngitis with penicillin V. Pediatric Infectious Diseases Journal 1991;10(2):126‐30. [DOI] [PubMed] [Google Scholar]
Howe 1997 {published and unpublished data}
- Howe RW, Millar MR, Coast J, Whitfield M, Peters TJ, Brookes S. A randomized controlled trial of antibiotics on symptom resolution in patients presenting to their general practitioner with a sore throat. British Journal of General Practice 1997;47(418):280‐4. [PMC free article] [PubMed] [Google Scholar]
Krober 1985 {published data only}
- Krober JW, Michels GN. Streptococcal pharyngitis: Placebo controlled double blind evaluation of clinical response to penicillin therapy. JAMA 1985;253(9):1271‐4. [DOI] [PubMed] [Google Scholar]
Landsman 1951 {published data only}
- Landsman JB, Grist NR, Black R, McFarlane D, Blair W. Sore throat in general practice. British Medical Journal 1951;1:326‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leelarasamee 2000 {published data only}
- Leelarasamee A, Leowattana W, Tobunluepop P, Chub‐upakarn S, Artavetakan W, Jarupoonphol V, et al. Amoxycillin for fever and sore throat due to non‐exudative pharyngotonsillitis: beneficial or harmful?. International Journal of Infectious Diseases 2000;4(2):70‐4. [DOI] [PubMed] [Google Scholar]
Little 1997 {published and unpublished data}
- Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997;315(7104):350‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997;314:722‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 1951 {published data only}
- MacDonald TC, Watson IH. Sulphonamides and acute tonsillitis: a controlled experiment in a Royal Airforce community. British Medical Journal 1951;1:323‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 1988 {published data only}
- Middleton DB, D'Amico F, Merenstein JH. Standardized symptomatic treatment versus penicillin as initial therapy for streptococcal pharyngitis. Journal of Pediatrics 1988;113(6):1089‐94. [DOI] [PubMed] [Google Scholar]
Nelson 1984 {published data only}
- Nelson JD. The effect of penicillin therapy on the symptoms and signs of streptococcal pharyngitis. Pediatric Infectious Disease 1984;3(1):10‐3. [DOI] [PubMed] [Google Scholar]
Petersen 1997 {published data only}
- Peterson K, Phillips RS, Soukup J, Komaroff AL, Aronson M. The effect of erythromycin on resolution of symptoms among adults with pharyngitis not caused by Group A Streptococcus. Journal of General Internal Medicine 1997;12(2):95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pichichero 1987 {published data only}
- Pichichero FA, Talpey WB, Green JL, Francis AB, Roghmann KJ, Hoekelman RA. Adverse and beneficial effects of immediate treatment of group A beta haemolytic streptococcal pharyngitis with penicillin. Pediatric Infectious Disease 1987;6(7):635‐43. [DOI] [PubMed] [Google Scholar]
Siegel 1961 {published data only}
- Siegel EE, Stollerman GH. Controlled studies of streptococcal pharyngitis in a pediatric population. New England Journal of Medicine 1961;265:559‐65. [Google Scholar]
Taylor 1977 {published data only}
- Taylor B, Abbott GD, McKerr M, Fergusson DM. Amoxycillin and co‐trimoxazole in presumed viral respiratory infections of childhood: placebo controlled trial. British Medical Journal 1977;2(6086):552‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wannamaker 1951 {published data only}
- Wannamaker LW, Rammelkamp CH, Denny FW, Brink WR, Houser HB, Hahn EO. Prophylaxis of acute rheumatic fever by treatment of the preceding streptococcal infection with various amounts of depot penicillin. American Journal of Medicine 1951;10:673‐94. [DOI] [PubMed] [Google Scholar]
Whitfield 1981 {published data only}
- Whitfield MJ, Hughes AO. Penicillin in sore throat. Practitioner 1981;225(1352):234‐9. [PubMed] [Google Scholar]
Zwart 2000 {published and unpublished data}
- Zwart S, Sachs AP, Rujis GJ, Gubbels JW, Hoes AW, Melker RA. Penicillin for acute sore throat: randomised double blind trial seven days versus three days treatment or placebo in adults. BMJ 2000;320(7228):150‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwart 2003 {published data only}
- Zwart S, Rovers MM, Melker RA, Hoes AW. Penicillin for acute sore throat in children: randomised, double blind trial. BMJ 2003;327(7427):1324‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Barwitz 1999 {published data only}
- Barwitz HJK. Common cold ‐ trial to rationalize management in general practice by recommendation [Erkaltung: eine Handlungsempfehlung]. Zeitschrift fur Allgemeinmedizin 1999;75:932‐8. [Google Scholar]
Bass 1986 {published data only}
- Bass J. Treatment of streptococcal pharyngitis revisited. JAMA 1986;256:740‐3. [PubMed] [Google Scholar]
Bishop 1952 {published data only}
- Bishop JM, Peden AS, Prankerd TAJ, Cawley RH. Acute sore throat. Clinical features, aetiology and treatment. Lancet 1952;1:1183‐7. [DOI] [PubMed] [Google Scholar]
Catanzaro 1958 {published data only}
- Catanzaro FJ, Chamovitz R. Prevention of rheumatic fever by treatment of streptococcal infections: factors responsible for failures. New England Journal of Medicine 1958;259:51‐7. [DOI] [PubMed] [Google Scholar]
Cruickshank 1960 {published data only}
- Cruickshank R. Sore throat: a controlled therapeutic trial in young adults. Controlled Clinical Trials: paper delivered at the conference convened by the Council for International Organisation of Medical Sciences. Oxford: Blackwell, 1960:38‐44.
Dowell 2001 {published data only}
- Dowell J, Pitkethly M, Bain J, Martin S. A randomised controlled trial of delayed antibiotic prescribing as a strategy for managing uncomplicated respiratory tract infection in primary care. British Journal of General Practice 2001;51:200‐5. [PMC free article] [PubMed] [Google Scholar]
Gerber 1985 {published data only}
- Gerber MA, Spadaccini LJ, Wright LL, Deutsch L, Kaplan EL. Twice‐daily penicillin in the treatment of streptococcal pharyngitis. American Journal of Diseases of Children 1985;139:1145‐8. [DOI] [PubMed] [Google Scholar]
Gerber 1989 {published data only}
- Gerber MA, Randolph MF. Failure of once‐daily penicillin V therapy for streptococcal pharyngitis. American Journal of Diseases of Children 1989;143:153‐5. [DOI] [PubMed] [Google Scholar]
Ginsburg 1980 {published data only}
- Ginsburg CM, McCracken GH, Crow SD, Steinberg JB, Cope F. A controlled comparative study of penicillin V and cefadroxil therapy on Group A streptococcal tonsillopharyngitis. Journal of International Medical Research 1980;8(Suppl 1):82‐6. [PubMed] [Google Scholar]
Guthrie 1988 {published data only}
- Guthrie RM, Ruoff GE, Rofman BA, Ginsberg D, Karp RR, Brown SM, et al. Aetiology of acute pharyngitis and clinical response to empirical therapy with erythromycin versus amoxicillin. Family Practice 1988;5:29‐35. [DOI] [PubMed] [Google Scholar]
Haverkorn 1971 {published data only}
- Haverkorn MV, Goslings WRO. Streptococcal pharyngitis in the general population. A controlled study of streptococcal pharyngitis and its complications in the Netherlands. Journal of Infectious Diseases 1971;124:339‐47. [DOI] [PubMed] [Google Scholar]
Herz 1988 {published data only}
- Herz MJ. Antibiotics and the adult sore throat ‐ an unnecessary ceremony. Family Practice 1988;5:196‐9. [DOI] [PubMed] [Google Scholar]
Howie 1970 {published data only}
- Howie JGR, Clark GA. Double‐blind trial of early demethylchlortetracycline in minor respiratory illness in general practice. Lancet 1970;Nov 28:1099‐102. [DOI] [PubMed] [Google Scholar]
Jensen 1991 {published data only}
- Jensen JH, Larsen SB. Treatment of recurrent acute tonsillitis with clindamycin. An alternative to tonsillectomy?. Clinical Otolaryngology 1991;16:498‐500. [DOI] [PubMed] [Google Scholar]
Kapur 2011 {published data only}
- Kapur A, Hannay D, Baxter G, Mitra A. A randomized prospective study to evaluate the natural history of acute upper respiratory infections in children and provide guidance for health professionals. American Journal of Respiratory and Clinical Care Medicine 2011;183:A4934. [Google Scholar]
Kolobukhina 2011 {published data only}
- Kolobukhina LV, Merkulova LN, Schelkanov MY, Isaeva EI, Kruzhkova IS, Malikov VE, et al. The efficacy of Ingavirin in the combined treatment of ARVI complicated by tonsillitis. Vestnik Otorinolaringologii 2011;6:91‐5. [PubMed] [Google Scholar]
Marlow 1989 {published data only}
- Marlow RA, Torrez AJ, Haxby D. The treatment of non streptococcal pharyngitis with erythromycin: a preliminary study. Family Medicine 1989;21:425‐7. [PubMed] [Google Scholar]
Massell 1951 {published data only}
- Massell BF, Sturgis GP, Knobloch JD, Streeper RB, Hall TN, Norcross P. Prevention of rheumatic fever by prompt penicillin therapy of hemolytic streptococcic respiratory infections. Journal of the American Medical Association 1951;146(16):1469‐74. [DOI] [PubMed] [Google Scholar]
McDonald 1985 {published data only}
- McDonald CJ, Tierny WM, Hui SL, French MLV, Leland DS, Jones RB. A controlled trial of erythromycin in adults with nonstreptococcal pharyngitis. Journal of Infectious Diseases 1985;152:1093‐4. [DOI] [PubMed] [Google Scholar]
Merenstein 1974 {published data only}
- Merenstein JH, Rogers KD. Early treatment and management by nurse practitioners. JAMA 1974;227:1278‐82. [DOI] [PubMed] [Google Scholar]
Morris 1956 {published data only}
- Morris AJ, Chamovitz R, Catanzaro FJ, Rammelkamp CH. Prevention of rheumatic fever by treatment of previous streptococcic infections; effect of sulfadiazine. Journal of the American Medical Association 1956;160(2):114‐6. [DOI] [PubMed] [Google Scholar]
Nasonova 1999 {published data only}
- Nasonova VA, Belov BS, Strachunsky LS, Sudilovskaya EI, Bogdanovich TM, Krechikova OI, et al. Antibacterial therapy of Streptococcus tonsillitis (quinsy and pharyngitis). Antibiotiki i Khimioterapiia 1999;44:19‐23. [PubMed] [Google Scholar]
Pandraud 2002 {published data only}
- Pandraud L. Therapeutic efficacy and clinical acceptability of fusafungine in follicular pharyngitis. Current Medical Research and Opinion 2002;18:381‐8. [DOI] [PubMed] [Google Scholar]
Randolph 1985 {published data only}
- Randolph MF, Gerber MA, DeMeo KK, Wright L. Effect of antibiotic therapy on the clinical course of streptococcal pharyngitis. Journal of Pediatrics 1985;106:870‐5. [DOI] [PubMed] [Google Scholar]
Schalen 1985 {published data only}
- Schalen L, Christenses P, Elisson I, Fex S, Kamme S, Schalen C. Inefficacy of penicillin V in acute laryngitis in adults. Evaluation from results of double‐blind study. Annals of Otology Rhinology and Laryngology 1985;94:14‐7. [DOI] [PubMed] [Google Scholar]
Schalen 1993 {published data only}
- Schalen L, Eliasson I, Kamme C, Schalen C. Erythromycin in acute laryngitis in adults. Annals of Otology Rhinology and Laryngology 1993;102:209‐14. [DOI] [PubMed] [Google Scholar]
Schwartz 1981 {published data only}
- Schwartz R, Wientzen RL, Pedeira F. Penicillin V for Group A Streptococcal pharyngotonsillitis. A randomised trial of seven vs ten days therapy. JAMA 1981;246:1790‐5. [DOI] [PubMed] [Google Scholar]
Shevrygin 2000 {published data only}
- Shevrygin BV, Manuilov BM. Therapeutic efficacy of new drug Pharingal at acute inflammatory diseases of pharynx and tonsils in pediatrics [Terapevticheskaia effektivnost' novogo perparata Faringal pri ostrykh vospalitel'nykh zabolevaniiakh glotki i mindalin u detei]. Antibiotiki i Khimioterapiia 2000;45:34‐6. [PubMed] [Google Scholar]
Shvartzman 1993 {published data only}
- Shvartzman P, Tabenkin H, Rosentzwaig A, Dolginov F. Treatment of streptococcal pharyngitis with amoxycillin once a day. BMJ 1993;306:1170‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stillerman 1986 {published data only}
- Stillerman M. Comparison of oral cephalosporins with penicillin for Group A streptococcal pharyngitis. Pediatric Infectious Diseases Journal 1986;5:648‐54. [DOI] [PubMed] [Google Scholar]
Stromberg 1988 {published data only}
- Stromberg A, Schwan A, Cars O. Five versus ten days treatment of group A streptococcal pharyngotonsillitis: a randomised controlled clinical trial with phenoxymethylpenicillin and cefadroxil. Scandinavian Journal of Infectious Disease 1988;20:36‐46. [DOI] [PubMed] [Google Scholar]
Supajatura 2012 {published data only}
- Supajatura V, Pongbaibul Y, Tharavichitkul P. Mangosteen spray for efficacious treatment of streptococcal pharyngitis. International Journal of Infectious Diseases 2012;16S:e441. [Google Scholar]
Todd 1984 {published data only}
- Todd JK, Todd N, Damato J, Todd WA. Bacteriology and treatment of purulent nasopharyngitis: a double blind, placebo‐controlled evaluation. Pediatric Infectious Disease 1984;3:226‐31. [DOI] [PubMed] [Google Scholar]
Valkenburg 1971 {published data only}
- Valkenburg HHMJ, Goslings WRO. Streptococcal pharyngitis in the general population. The attack rate if not treated with penicillin. Journal of Infectious Diseases 1971;124:783‐6. [DOI] [PubMed] [Google Scholar]
Additional references
ABS 1985
- Australian Bureau of Statistics. Australian Health Survey. Canberra: AGPS: (Cat No 4311.0) 1985:11‐52.
Del Mar 1992a
- Mar C. Managing sore throat: a literature review. I. Making the diagnosis. Medical Journal of Australia 1992;156:572‐5. [PubMed] [Google Scholar]
Del Mar 1992b
- Mar C. Managing sore throats: a literature review. II. Do antibiotics confer benefit?. Medical Journal of Australia 1992;156:644‐9. [PubMed] [Google Scholar]
Del Mar 1992c
- Mar C. Spontaneously remitting disease, principles of management. Medical Journal of Australia 1992;157:101‐7. [PubMed] [Google Scholar]
Froom 1990
- Froom J, Culpepper L, Grob P, Bartelds A, Bowers P, Bridges‐Webb C, et al. Diagnosis and antibiotic treatment of acute otitis media: report from International Primary Care Network. BMJ 1990;300:582‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goslings 1963
- Goslings WRO, Valkenberg HA, Bots AW, Lorrier JC. Attack rates of streptococcal pharyngitis, rheumatic fever and glomerulonephritis in the general population. A controlled pilot study of controlled streptococcal pharyngitis in one village. New England Journal of Medicine 1963;268:687‐94. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horder 1954
- Horder J, Horder E. Illness in general practice. Practitioner 1954;173:177‐87. [PubMed] [Google Scholar]
Howie 1971
- Howie JGR, Gill G, Durno D. Respiratory illness and antibiotic use in general practice. Journal of the Royal College of General Practitioners 1971;21:657‐61. [PMC free article] [PubMed] [Google Scholar]
Howie 1978
- Howie JGR. Antibiotics and respiratory illness in general practice: prescribing policy and workload. British Medical Journal 1978;2:1342‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howie 1985
- Howie JGR, Foggo B. Antibiotics, sore throats and rheumatic fever. Journal of the Royal College of General Practitioners 1985;35:223‐4. [PMC free article] [PubMed] [Google Scholar]
Keith 2010
- Keith T, Saxena S, Murray J, Sharland M. Risk‐benefit analysis of restricting antimicrobial prescribing in children: what do we really know?. Current Opinion in Infectious Disease 2010;23:242‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester: Wiley‐Blackwell, 2011. [Google Scholar]
Reveiz 2013
- Reveiz L, Cardona AF. Antibiotics for acute laryngitis in adults. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD004783.pub4] [DOI] [PubMed] [Google Scholar]
van Driel 2013
- Driel ML, Sutter AI, Keber N, Habraken H, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD004406.pub3] [DOI] [PubMed] [Google Scholar]
Venekamp 2013
- Venekamp RP, Sanders S, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000219.pub3] [DOI] [PubMed] [Google Scholar]
Wessels 2011
- Wessels MR. Streptococcal pharyngitis. New England Journal of Medicine 2011;364:648‐55. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Del Mar 1997
- Mar CB, Glasziou PP, Hayem M. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 1997, Issue 2. [DOI: 10.1002/14651858.CD000023.pub3] [DOI] [PubMed] [Google Scholar]
Del Mar 2000
- Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000023.pub3] [DOI] [PubMed] [Google Scholar]
Del Mar 2004
- Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000023.pub3] [DOI] [PubMed] [Google Scholar]
Del Mar 2006
- Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD000023.pub3] [DOI] [PubMed] [Google Scholar]
Spinks 2009
- Spinks AB, Glasziou PP, Mar CB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD000023.pub3] [DOI] [PubMed] [Google Scholar]
Spinks 2011
- Spinks AB, Glasziou PP, Mar CB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD000023.pub3] [DOI] [PubMed] [Google Scholar]



