A new marine fossil assemblage from the Early Triassic shows unexpected phyletic diversity and functional complexity.
Keywords: End-Permian mass extinction, biotic recovery, Early Triassic, marine ecosystems, Idaho, Paris Biota
Abstract
In the wake of the end-Permian mass extinction, the Early Triassic (~251.9 to 247 million years ago) is portrayed as an environmentally unstable interval characterized by several biotic crises and heavily depauperate marine benthic ecosystems. We describe a new fossil assemblage—the Paris Biota—from the earliest Spathian (middle Olenekian, ~250.6 million years ago) of the Bear Lake area, southeastern Idaho, USA. This highly diversified assemblage documents a remarkably complex marine ecosystem including at least seven phyla and 20 distinct metazoan orders, along with algae. Most unexpectedly, it combines early Paleozoic and middle Mesozoic taxa previously unknown from the Triassic strata, among which are primitive Cambrian-Ordovician leptomitid sponges (a 200–million year Lazarus taxon) and gladius-bearing coleoid cephalopods, a poorly documented group before the Jurassic (~50 million years after the Early Triassic). Additionally, the crinoid and ophiuroid specimens show derived anatomical characters that were thought to have evolved much later. Unlike previous works that suggested a sluggish postcrisis recovery and a low diversity for the Early Triassic benthic organisms, the unexpected composition of this exceptional assemblage points toward an early and rapid post-Permian diversification for these clades. Overall, it illustrates a phylogenetically diverse, functionally complex, and trophically multileveled marine ecosystem, from primary producers up to top predators and potential scavengers. Hence, the Paris Biota highlights the key evolutionary position of Early Triassic fossil ecosystems in the transition from the Paleozoic to the Modern marine evolutionary fauna at the dawn of the Mesozoic era.
INTRODUCTION
The Permian-Triassic boundary (PTB) [~251.9 million years ago (Ma)] is characterized by the largest Phanerozoic mass extinction, marking the end of the dominance of the Sepkoski’s Paleozoic evolutionary fauna and the expansion of the Modern evolutionary fauna (1). The PTB and subsequent Early Triassic recovery interval are characterized by recurrent marked changes in water temperature (2, 3), large-scale fluctuations of the global carbon cycle, and harsh marine conditions including a combination of ocean acidification, anoxia, euxinia, and fluctuating productivity (Fig. 1) (4–6).
Fig. 1. Chronostratigraphic subdivisions of the Early Triassic [radiometric ages from studies by Galfetti et al. (5), Ovtcharova et al. (47), Ovtcharova et al. (48), and Burgess et al. (49)] with simplified global geochemical trends [δ13Ccarb data from Galfetti et al. (5); anoxic episodes modified following studies by Grasby et al. (6), Galfetti et al. (50), Ware et al. (51), and Hermann et al. (52)] and the relative temperature fluctuations in the Tethyan realm [adapted from studies by Sun et al. (2) and Romano et al. (3)].
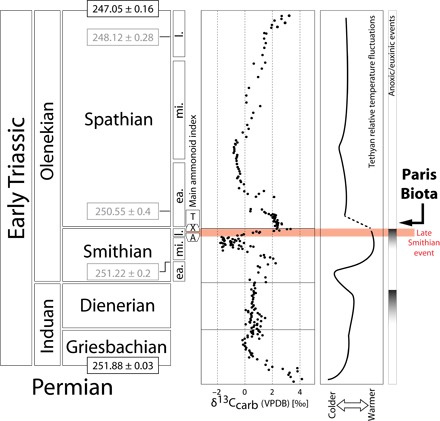
Subdivisions of the Smithian and Spathian follow the ammonoid zonation from studies by Brühwiler et al. (53) and Guex et al. (54). The late Smithian extinction event is highlighted in red. Time correlations for the late Smithian and the Smithian-Spathian boundary are based on high-resolution ammonoid zonations (20, 55, 56). A, Anasibirites beds; X, Xenoceltitidae beds (both late Smithian); T, Tirolites beds (early Spathian); ea., early; mi., middle; l., late; VPBD, Vienna Pee Dee belemnite.
The postcrisis biotic recovery was rapid for some nektonic-pelagic groups, such as ammonoids and conodonts (7, 8). In contrast, delayed, spatially heterogeneous recovery of species-poor communities is assumed to be the hallmark of benthic ecosystems (9–16).
Until now, the oldest known marine Mesozoic complex ecosystem was dated from the Middle Triassic of China (Luoping biota, ~242 Ma) (17). Here, we report a new, exceptionally well-preserved and diversified Early Triassic marine biota from Paris, southeastern Idaho, USA (see Figs. 1 to 3 and the Supplementary Materials). Ammonoid and conodont biostratigraphy indicates an earliest Spathian (middle Olenekian, ~250.6 Ma) (Fig. 1) (5) age for this biota and is therefore the first and oldest known Early Triassic complex marine ecosystem.
Fig. 3. Simplified stratigraphic log showing main Early Triassic fossil-bearing levels currently known in the Bear Lake area.
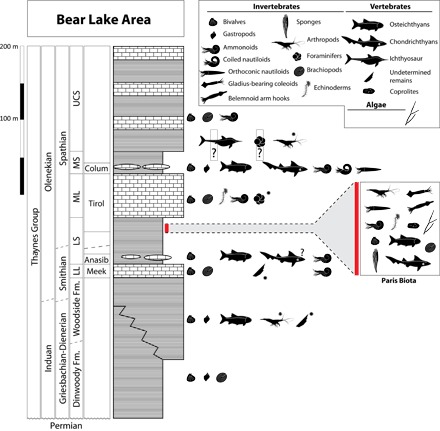
Fossil occurrence is modified following the study by Romano et al. (57), with additional data from studies by Massare and Callaway (58) (ichthyosaur remains), Tosk and Andersson (59) (foraminifers), and Hofmann et al. (60) and from our own field observations (black stars). Conodonts are also sparsely present throughout the section but are not represented here. Stratigraphy follows the main units defined by Kummel (61, 62). Zonation is based on ammonoids (20, 54, 55, 62, 63). Position of the Paris Biota is highlighted in red. A U-Pb age of 250.55 ± 0.4 My is associated with the Tirolites/Columbites beds from South China (5). LL, Lower Limestone; LS, Lower Shale; ML, Middle Limestone; MS, Middle Shale; UCS, Upper Calcareous Siltstone; Meek, Meekoceras beds; Anasib, Anasibirites beds; Tirol, Tirolites beds; Colum, Columbites beds.
The earliest Spathian represents a time of transition following a severe late Smithian extinction event (Fig. 1) (7, 8). During the Early Triassic recovery, the middle Smithian and late Smithian were subject to renewed, large-scale perturbations of the global biogeochemical cycles (4–6, 18), including some of the largest δ13C excursions of the Phanerozoic, and a peak in seawater temperatures (Fig. 1) (2, 3). In low latitudes, these high temperatures, along with anoxia, are hypothesized to have been lethal to many marine clades (2). The newly discovered Paris Biota (named herein) is therefore highly unexpected because it documents a markedly diversified benthic ecosystem only ~1.3 million years (My) after the PTB in an equatorial setting.
Exposures and sampled material
The Paris Biota was found in four neighboring and equivalent exposures of the upper part of the early Spathian Lower Shale unit of the Early Triassic Thaynes Group [sensu Lucas et al. (19)], west of the city of Paris, Idaho (see Figs. 2 and 3, figs. S1 and S2, and the Supplementary Materials). The alternating limestones and shales of the Thaynes Group reflect deposition within the relatively shallow western U.S. basin. During the Early Triassic, it was located at a near-equatorial position on the western margin of Pangea (Fig. 2C). Tirolites ammonoid specimens are found throughout the four studied exposures of the upper part of the Lower Shale unit, confirming an earliest Spathian age for this biota (see the Supplementary Materials).
Fig. 2. Maps showing the location of the newly discovered Paris Biota.
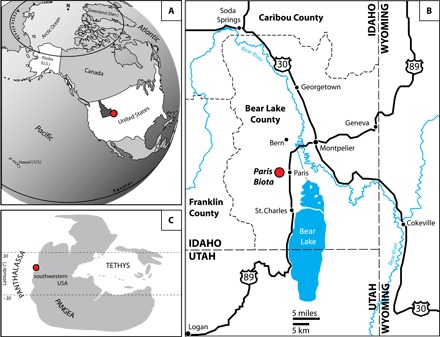
(A) Present-day map showing the location of southeastern Idaho in western United States. (B) Map of southeastern Idaho showing the location of the Paris Biota. (C) Early Triassic map with the location of the Paris Biota. (A) to (C) were modified following the study by Romano et al. (57).
Compared to previously described Early Triassic assemblages [for example, see study by Schubert and Bottjer (9)], the Paris Biota shows a remarkably high diversity (Figs. 4 to 6, figs. S3 and S4, and table S1) with abundant sponges, brachiopods, bivalves, ammonoids, belemnoids, arthropods, and fishes. Ammonoids and bivalves dominate the biota in terms of abundance, as is normally observed in Smithian and Spathian fossil levels from the western U.S. basin (13, 20). Crinoids, ophiuroids, orthoconic nautiloids, gladius-bearing coleoids, fishes, algae, and vertebrate coprolites also occur. Overall, more than 750 individuals (excluding isolated fossil pieces and fragments) representing at least seven phyla and >20 orders have been collected so far (Figs. 4 to 6, figs. S3 to S28, and table S1). Some of these organisms are documented from the Early Triassic for the first time (leptomitid sponges and gladius-bearing coleoids), and several display anatomical characters that were thought to have evolved much later (for example, echinoderms), indicating an early and rapid post-PTB diversification for these groups as well as previously unknown phylogenetical links between Paleozoic and Mesozoic taxa.
Fig. 4. Characteristic earliest Spathian organisms of the Paris Biota.

(A) Leptomitid protomonaxonid sponge (s) and ammonoids (a) (slab IMNH IP-026/777). (B) Leptomitid protomonaxonid sponge showing a twisted apex and minute epizoan brachiopods (arrows) (slab UBGD 30505). (C) Glyphidean lobster Litogaster ?turnbullensis (UBGD 30548). (D) New thylacocephalan genus (UBGD 30561). (E) Penaeid shrimp (IMNH IP-026/778). (F) Caridean shrimp under ultraviolet (UV) light (365 nm) (UBGD 30558). (G) Gladius-bearing coleoid (UBGD 30545). (H) Holocrinid crinoid stem with cirri (UBGD 30563). (I) Ophiuroid (UBGD 30565). Scale bars, 5 mm (A and C to I) and 10 mm (B). [Photo credits: A. Brayard, Université Bourgogne Franche-Comté (A to G); T. Saucède, Université Bourgogne Franche-Comté (H); and B. Thuy, Natural History Museum Luxembourg (I).]
Fig. 6. Characteristic earliest Spathian organisms of the Paris Biota.
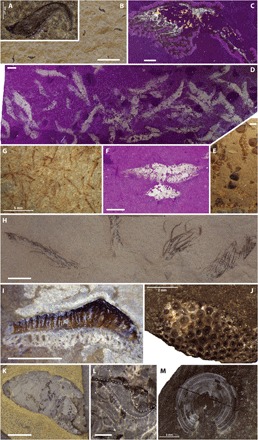
(A and B) Belemnoid arm hooks (UBGD 30540 and 30544). (C) Penaeid shrimp (UBGD 30559). (D to F) Mass accumulations of caridean shrimps under UV light (365 nm) and natural light (slabs UBGD 30553 and 30554). (G) Rod-shaped, unbranched specimens of putative noncalcified dasycladales (slab UBGD 30576). (H) Isolated arms of a holocrinid crinoid (UBGD 30563). (I) Chondrichthyan tooth referable to Acrodus (slab IMNH 1143/46168). (J) Osteichthyan tooth plate (slab UBGD 30569). (K) Coprolite specimen (UBGD 30573). (L) Closeup view of belemnoid hooks accumulated in a coprolite (slab UBGD 30575). (M) Discinoid inarticulate brachiopod Orbiculoidea sp. (UBGD 30522). Scale bars, 5 mm (A to K and M) and 0.5 mm (L). [Photo credits: A. Brayard, Université Bourgogne Franche-Comté (A to H and J to M); L. J. Krumenacker, Montana State University (I).]
There is no major faunal difference between the four sampled exposures, suggesting similar ecological conditions and depositional settings. Macroscopic field observations and thin section analyses indicate that a part of the sedimentary succession was formed by storm-mobilized sediments. Abundant planar and undulated laminae indicate an outer shelf in the deepest part of the storm-dominated environments (that is, 60 to 100 m below sea level; see the Supplementary Materials). The completeness of delicate specimens, especially echinoderms and crustaceans, demonstrates that they were usually entombed rapidly and close to their original habitat. There is no evidence of any size-sorting, mixing, or reworking processes. Rarity of infaunal bioturbation, high sedimentation rate, and abundance of framboidal and polyhedral pyrite suggest upper sediment dysoxic to anoxic conditions. However, the seafloor was oxygenated, as testified by the abundance of benthic organisms including crinoids and ophiuroids, which require well-oxygenated conditions.
Ammonoid, sponge, arthropod, and brachiopod specimens are usually flattened. Most other fossils are compressed but preserve some three-dimensional features (Figs. 4 to 6 and figs. S5 to S28). Contrary to some Middle Triassic assemblages preserving soft parts [for example, see study by Hu et al. (17)], no evidence of microbial mat–enhanced preservation was found. Mollusks (except gladius-bearing coleoids) and echinoderms are preserved in calcite. Sponges, arthropods, brachiopods, and coprolites are preserved in calcium phosphate (figs. S29 to S33), suggesting rapid decay and burial in phosphatizing microenvironments. Phosphatic preservation is common for coprolites, vertebrate remains, cuticle of crustaceans, and coleoid soft parts (21) but rare for sponges [for example, see study by Castellani et al. (22)]. Belemnoid hooks and parts of gladius-bearing coleoids are preserved as carbonaceous structures (fig. S32).
RESULTS
The Paris Biota is highly diversified and shows a largely unexpected composition. It combines taxa usually found in the Early Triassic from the western U.S. basin (for example, ammonoids, nautiloids, bivalves, lingulids, ophiuroids, and vertebrates) with more unusual taxa and with others previously unknown for this time interval (leptomitid sponges, epizoan brachiopods, crinoids, gladius-bearing coleoids, belemnoids, arthropods, and algae) (Figs. 4 to 6 and figs. S5 to S28). In this remarkable biota, leptomitid protomonaxonids (Figs. 4, A and B, and 5 and figs. S5 to S11) are by far the most unexpected taxon. Although isolated spicules and specimens of Early Triassic siliceous and hypercalcified sponges are frequently found in the western U.S. basin (23–28), all complete sponge specimens recovered from the Paris Biota belong to the leptomitid protomonaxonids. These sponges differ markedly from all other sponge taxa reported so far from the Early Triassic, including the western U.S. basin (24–26). In particular, they show a weak and delicate structure characterized by primarily longitudinal spicules, a helical twisting of the skeleton, and a fringe of spicules projecting from the apex to form marginalia (Fig. 5 and figs. S5 to S11). These features are typical of leptomitid protomonaxonids and are unknown from other sponge groups, including the morphologically plastic, often ecophenotypically variable demosponges. Convergence with any other sponge group can therefore be confidently discarded, including the grossly reminiscent modern hexactinellid sponge Euplectella aspergillum, whose skeleton is primarily made of a fused (as adults), orthogonally reticulate grid that is reinforced by diagonal (helical) bundles and an oscular sieve plate (see the Supplementary Materials) (29). Leptomitid protomonaxonids are phylogenetically distant from extant siliceous sponges and are most typical of Sepkoski’s Cambrian evolutionary fauna (1), most particularly of the Cambrian Burgess Shale–type faunas, although these protomonaxoids are also present in lower abundance in some Ordovician deposits with exceptional preservation (30–32). Some Silurian faunas may be dominated by protomonaxonids but without clear links to older faunas (30). Very few protomonaxonids from later Paleozoic rocks and no examples of Leptomitidae are known. Therefore, the Paris Biota sponges represent a Lazarus taxon with a ~200-My gap in the fossil record, highlighting the poor resolution of the fossil record for this group (30). Long ghost lineages for surviving Cambrian-type sponges are also known elsewhere, including a Late Triassic calcareous sponge genus previously restricted to the Cambrian (33). The minute epizoan brachiopods (Figs. 4B and 5C and fig. S13) occasionally found in close association with these leptomitid sponges directly echo similar biotic associations described in early Paleozoic biotas (34, 35), suggesting that this brachiopod-sponge association also survived ~200 My longer than previously thought. Furthermore, these specimens indicate that Early Triassic brachiopods exploited not only seafloor habitats but also additional tiering levels above the substratum.
Fig. 5. Specimens and characteristic features of leptomitid protomonaxonid sponges from the Paris Biota.

(A and B) General and closeup view of the specimen UBGD 30504 showing projected longitudinal spicules (ls) from the apex forming a fringe of marginalia (m) and transverse spicules (ts). (C and D) Closeup views of twisted apex (ta) of two specimens (UBGD 30505 and 30581) under natural and UV light (365 nm). Projecting spicules from the apex forming a fringe of marginalia are also visible. Fine transverse spicules appear mainly as wrinkles, perpendicular to the longitudinal spicules. An epizoan brachiopod (e) is attached to the sponge specimen C. (E and F) Closeup views of specimens UBGD 30506 and 30508, showing longitudinal and transverse spicules. (G and H) Large-sized specimens UBGD 30510 and 30511. Scale bars, 5 mm (A to D and G and H), 2 mm (E), and 1 mm (I). [Photo credits: A. Brayard, Université Bourgogne Franche-Comté.]
New forms of the articulate crinoid order Holocrinida are documented for the first time in the Early Triassic, with most skeletal elements articulated (Figs. 4H and 6H and figs. S22 and S23). We collected two proximal stems with cirri, and subcomplete isolated arms. These fossils display advanced characters (for example, presence of cryptosymplectial articulations; see the Supplementary Materials), indicating an intense morphological diversification before or during the earliest Spathian, much earlier than previously thought (36, 37). Ophiuroid remains include one complete specimen, which is considerably larger than previously known Early Triassic ophiuroids (Fig. 4I and figs. S24 and S25) (38, 39). The diagnostic skeletal characters (for example, spine articulations) suggest basal ophiodermatid affinities, thus pushing the origin of the ophiodermatid clade and the early ophiuroid diversification to the lower limits of divergence time estimates (40, 41).
We also report the unexpected occurrence of gladius-bearing coleoids (Fig. 4G and fig. S17). Previously unknown in Early Triassic strata, gladius-bearing coleoids diversified during the Jurassic and the Cretaceous [for example, see study by Fuchs and Larson (42)]. The specimens reported here demonstrate that these gladius-bearing coleoids already existed by the Early Triassic. A putative representative of this clade was recently described from the Early Permian (43) but with a gladius morphology completely distinct from Mesozoic ones. Proostracum-bearing coleoids are classically considered as the most likely ancestors of gladius-bearing coleoids (44). Nevertheless, regardless of the still-unknown phylogenetic relationships between Permian and Triassic taxa, the microlaminated ultrastructure and organic composition of the Paris Biota gladii suggest that gladius-bearing coleoids did not evolve from proostracum-bearing coleoids. Instead, they indicate an independent evolution of these clades from a still-unknown late Paleozoic common ancestor (see the Supplementary Materials).
Hundreds of mostly isolated belemnoid arm hooks have also been recovered (Fig. 6, A and B, and fig. S16). Similar forms were already described from a Spitsbergen Spathian locality (45); thus, their occurrence in the Paris Biota is not surprising per se, although their high abundance is notable. The size of these hooks suggests that their bearers were about 15 to 20 cm long, corresponding to middle-sized predators. Their abundance and stratigraphic recurrence indicate a stable presence and high life abundance of these predators in the earliest Spathian of this area. In addition, hook concentrations observed in some coprolites (Fig. 6, K and L) demonstrate that these active predators were consumed by even larger vertebrate predators (fishes or reptiles), the fossils of which are known from the same area (see Fig. 6, I and J, and the Supplementary Materials).
At least five taxa of arthropods are present in the Paris Biota (Figs. 4, C to F, and 6, C to F). These include two new genera of thylacocephalans, an uncommon and enigmatic group of arthropods (Fig. 4D and fig. S21). This is the first reported occurrence of thylacocephalans from Triassic rocks in North America, considerably extending their spatiotemporal distribution (46). In addition, the Paris Biota yields the richest fauna of Triassic crustaceans found so far in North America, with abundant decapods including glyphidean lobsters and caridean and penaeid shrimps [Figs. 4, C to F, and 6, C to F, and figs. S18 to S21].
Last, algae are represented by rod-shaped and branching morphotypes showing potential affinities with noncalcified dasycladales and other late Paleozoic green algae, respectively (Fig. 6G and fig. S28). They cover large surfaces at some horizons, suggesting transient blooms. These indicate that algae may have been important primary producers in some Early Triassic trophic networks. The Early Triassic diversity, abundance, and geographic distribution of these fragile organisms (especially when noncalcified) remain largely unknown.
DISCUSSION
Overall, the Paris Biota illustrates a diversified and trophically complete marine ecosystem—from primary producers up to top predators and potential scavengers (see figs. S3 to S28 and the Supplementary Materials). It is close in complexity to the Middle Triassic Luoping biota (17), which has classically been viewed as an iconic example of a post-PTB fully rediversified marine ecosystem. This is all the more notable given the fact that the Paris Biota lived in the immediate aftermath of the end-Smithian extinction event in an equatorial setting, that is, at a time and place where marine ecosystems are thought to be heavily depauperate (2).
The unexpected co-occurrence of taxa previously known only in early Paleozoic or in middle-to-late Mesozoic strata demonstrates that some Early Triassic ecosystems were much more phylogenetically diverse and functionally much more complex than previously thought. The Paris Biota illustrates the oldest occurrence of derived characters in several clades (fig. S3), and it shows that at least some Early Triassic marine communities include ancient lineages in the lowest trophic levels together with newly evolved groups occupying higher trophic levels.
By revealing previously hidden ecosystem complexity and unexpected taxonomic occurrences that increase global Early Triassic biodiversity, this remarkable biota constitutes a new landmark for understanding the marine recovery dynamics after the end-Permian mass extinction. It stands in stark contrast with previous works that suggested a sluggish recovery and low diversity of marine benthic organisms during the Early Triassic (9, 10). The Paris Biota shows that functionally complex, trophically multileveled marine ecosystems were actually present soon (~1.3 My) after the end-Permian mass extinction, at least in some areas that may have acted as biotic refugia. However, its peculiar composition indicates that its high diversity is not the simple consequence of a rapid post-PTB diversification. Instead, it represents the combined effect of the maintenance of long-ranging Paleozoic taxa and the early appearance of derived clades whose minimum age of origination is pre-Spathian (see the Supplementary Materials). The detailed timing of this faunal transition remains unknown because Lagerstätten (that is, sites of exceptional preservation) are extremely rare in the Permian-Triassic interval and they provide only a few macroevolutionary calibration points. The frequent low-diversity benthic assemblages commonly sampled in Early Triassic rocks were shown to have a biased composition for some major benthic clades (15, 25). They might therefore represent poorly sampled, taxonomically biased subsets of much more diversified ecosystems resembling the Paris Biota, at least at the level of the western U.S. basin. Alternatively, the Paris Biota may actually represent an assemblage whose taxonomic and functional diversity results from peculiar biotic and environmental conditions acting at the local scale. Additional field data from correlative beds and different depositional environments are required before general conclusions can be drawn on the spatiotemporal distribution of the Paris Biota. Nevertheless, this finding illustrates that the Late Permian–Early Triassic fossil record remains incompletely known for many marine higher-level taxa, even in the intensively studied western U.S. basin that is probably one of the best studied areas in the world for this time interval. Consequently, conclusions of a uniform recovery at the basin scale are no more supportable on the basis of currently available evidences, than would be a simple recovery model at the global scale.
Revealing the link between community composition and recovery dynamics will be critical in understanding the transition from the Paleozoic to the Modern evolutionary faunas. The Paris Biota highlights the key evolutionary position of Early Triassic fossil ecosystems in the transition from the Paleozoic to the Modern biosphere and that the rise of the Modern evolutionary fauna at least sometimes emerged from taxonomically, phylogenetically, and ecologically diverse communities.
MATERIALS AND METHODS
The four exposures studied are located near the city of Paris, southeastern Idaho (see Fig. 2 and the Supplementary Materials). More than 750 (sub)complete individual specimens (excluding isolated fossil pieces and fragments) were collected from these exposures so far. Figured specimens were housed in the collections of the Université de Bourgogne (Dijon, France) and the Idaho Museum of Natural History (Pocatello, USA). Specimens were observed in natural light using a Leica M205C binocular microscope coupled with a Leica DFC295 digital camera. Photographs were taken in natural light and UV light (365 nm) using a Nikon D5300 reflex camera and processed in Adobe Photoshop CS5. A camera lucida attached to Leica M205C was used for interpretative drawings. Scanning electron microscope (SEM) observations coupled with energy-dispersive spectrometer (EDS) analyses were performed at the Institut Carnot de Bourgogne (Dijon, France) to determine the elemental composition of fossil fragments. Variably oriented thin sections were also prepared and observed in natural and polarized light microscopy using a Nikon AZ100 microscope coupled with a digital camera.
Supplementary Material
Acknowledgments
We thank H. Hagdorn, L. E. Holmer, A. H. Knoll, A. Pisera, T. M. Scheyer, and D. Vachard for discussion. L.J.K. thanks C. Powell for assistance in the field. We acknowledge the Bear Lake County Road department and D. M. Clow (Ogden) for allowing access to the studied exposures. We also thank the reviewers for their constructive comments. Funding: This work is a contribution to the Agence Nationale de la Recherche projects AFTER (ANR-13-JS06-0001-01) and EvoDevOdonto (ANR-14-ACHN-0010). Author contributions: Fieldwork was carried out by A.B., L.J.K., J.F.J., K.G.B., E.F., E.V., N.O., N.G., D.A.S, and G.E. Thin section studies were performed by E.V. and N.O. Determinations of ammonoids: A.B., J.F.J., and K.G.B.; nautiloids: A.B.; belemnoid hooks: A.B. and K.G.B.; gladius-bearing coleoids: L.D.; sponges: J.P.B.; crinoids: T.S.; ophiuroids: B.T.; arthropods: S.C.; brachiopods: A.B.; bivalves: M.H.; conodonts: N.G.; other vertebrate remains: C.R.; algae: A.B. and G.E. All authors contributed to the discussion. A.B., E.F., N.G., E.V., and G.E. wrote the paper, with contribution from all other authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Figured specimens were housed at the Université de Bourgogne, Dijon, France, and at the Idaho Museum of Natural History, USA.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/2/e1602159/DC1
Supplementary Text
fig. S1. Field view of the upper part of the Lower Shale unit containing the Paris Biota.
fig. S2. Log section of the main Paris Biota exposure.
fig. S3. Taxa of the Paris Biota showing unexpected or extended temporal distributions, new Early Triassic spatiotemporal occurrences, new oldest records of derived characters for the clade, and new ecologic or trophic interactions.
fig. S4. Artistic reconstruction of the Paris Biota.
fig. S5. Closeup view of an apex of a leptomitid protomonaxonid sponge.
fig. S6. Closeup views of twisted apex of two leptomitid protomonaxonid sponges.
fig. S7. Closeup views of longitudinal and transverse spicules of four leptomitid protomonaxonid sponges.
figs. S8 to S10. Leptomitid protomonaxonid sponges from the Paris Biota.
fig. S11. Leptomitid protomonaxonid sponge from the Paris Biota showing attachment to a shell fragment.
fig. S12. Linguloid and discinoid inarticulate brachiopods.
fig. S13. Sponge specimen showing minute epizoan brachiopods and closeup views of epizoan brachiopods.
fig. S14. Bivalve specimens.
fig. S15. Ammonoid and nautiloid specimens.
fig. S16. Belemnoid arm hooks.
fig. S17. Gladius-bearing coleoid.
fig. S18. Different types of preservation for crustaceans from the Paris Biota.
fig. S19. Penaeidean and caridean shrimps and accumulations.
fig. S20. Penaeid shrimps, caridean shrimp, and litogastrid lobsters.
fig. S21. Thylacocephalan specimens.
fig. S22. Holocrinid specimens.
fig. S23. Closeup view of a crinoid stem with cirri and closeup view of an isolated pinnulate arm.
fig. S24. Ophiuroid specimens.
fig. S25. Closeup view of the spine articulations of an ophiuroid specimen.
fig. S26. Vertebrate remains from the Paris Biota.
fig. S27. Coprolites from the Paris Biota.
fig. S28. Algal morphotypes from the Paris Biota.
fig. S29 and S30. SEM photographs and EDS analyses for leptomitid protomonaxonid sponge specimens.
fig. S31. SEM photograph and EDS analysis for a crustacean specimen.
fig. S32. SEM photograph and EDS analysis for a belemnoid hook specimen.
fig. S33. SEM photograph and EDS analyses for a leptomitid protomonaxonid sponge specimen.
table S1. Sampled taxa from the Paris Biota.
REFERENCES AND NOTES
- 1.Sepkoski J. J., Jr, A factor analytic description of the Phanerozoic marine fossil record. Paleobiology 7, 36–53 (1981). [Google Scholar]
- 2.Sun Y., Joachimski M. M., Wignall P. B., Yan C., Chen Y., Jiang H., Wang L., Lai X., Lethally hot temperatures during the Early Triassic greenhouse. Science 338, 366–370 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Romano C., Goudemand N., Vennemann T. W., Ware D., Schneebeli-Hermann E., Hochuli P. A., Brühwiler T., Brinkmann W., Bucher H., Climatic and biotic upheavals following the end-Permian mass extinction. Nat. Geosci. 6, 57–60 (2013). [Google Scholar]
- 4.Payne J. L., Lehrmann D. J., Wei J., Orchard M. J., Schrag D. P., Knoll A. H., Large perturbations of the carbon cycle during recovery from the end-Permian extinction. Science 305, 506–509 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Galfetti T., Bucher H., Ovtcharova M., Schaltegger U., Brayard A., Brühwiler T., Goudemand N., Weissert H., Hochuli P. A., Cordey F., Guodun K. A., Timing of the Early Triassic carbon cycle perturbations inferred from new U–Pb ages and ammonoid biochronozones. Earth Planet. Sci. Lett. 258, 593–604 (2007). [Google Scholar]
- 6.Grasby S. E., Beauchamp B., Embry A., Sanei H., Recurrent Early Triassic ocean anoxia. Geology 41, 175–178 (2013). [Google Scholar]
- 7.Brayard A., Escarguel G., Bucher H., Monnet C., Brühwiler T., Goudemand N., Galfetti T., Guex J., Good genes and good luck: Ammonoid diversity and the end-Permian mass extinction. Science 325, 1118–1121 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Orchard M. J., Conodont diversity and evolution through the latest Permian and Early Triassic upheavals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 93–117 (2007). [Google Scholar]
- 9.Schubert J. K., Bottjer D. J., Aftermath of the Permian-Triassic mass extinction event: Paleoecology of Lower Triassic carbonates in the western USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 116, 1–39 (1995). [Google Scholar]
- 10.Twitchett R. J., Wignall P. B., Trace fossils and the aftermath of the Permo-Triassic mass extinction: Evidence from northern Italy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 124, 137–151 (1996). [Google Scholar]
- 11.Twitchett R. J., Krystyn L., Baud A., Wheeley J. R., Richoz S., Rapid marine recovery after the end-Permian mass-extinction event in the absence of marine anoxia. Geology 32, 805–808 (2004). [Google Scholar]
- 12.Beatty T. W., Zonneveld J.-P., Henderson C. M., Anomalously diverse Early Triassic ichnofossil assemblages in northwest Pangea: A case for a shallow-marine habitable zone. Geology 36, 771–774 (2008). [Google Scholar]
- 13.Hofmann R., Hautmann M., Brayard A., Nützel A., Bylund K. G., Jenks J. F., Vennin E., Olivier N., Bucher H., Recovery of benthic marine communities from the end-Permian mass extinction at the low latitudes of eastern Panthalassa. Palaeontology 57, 547–589 (2014). [Google Scholar]
- 14.Hautmann M., Bagherpour B., Brosse M., Frisk Å., Hofmann R., Baud A., Nützel A., Goudemand N., Bucher H., Competition in slow motion: The unusual case of benthic marine communities in the wake of the end-Permian mass extinction. Palaeontology 58, 871–901 (2015). [Google Scholar]
- 15.Brayard A., Meier M., Escarguel G., Fara E., Nützel A., Olivier N., Bylund K. G., Jenks J. F., Stephen D. A., Hautmann M., Vennin E., Bucher H., Early Triassic Gulliver gastropods: Spatio-temporal distribution and significance for biotic recovery after the end-Permian mass extinction. Earth Sci. Rev. 146, 31–64 (2015). [Google Scholar]
- 16.Foster W. J., Danise S., Sedlacek A., Price G. D., Hips K., Twitchett R. J., Environmental controls on the post-Permian recovery of benthic, tropical marine ecosystems in western Palaeotethys (Aggtelek Karst, Hungary). Palaeogeogr. Palaeoclimatol. Palaeoecol. 440, 374–394 (2015). [Google Scholar]
- 17.Hu S.-x., Zhang Q.-y., Chen Z.-Q., Zhou C.-y., Lü T., Xie T., Wen W., Huang J.-y., Benton M. J., The Luoping biota: Exceptional preservation, and new evidence on the Triassic recovery from end-Permian mass extinction. Proc. Biol. Sci. 278, 2274–2282 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomazo C., Vennin E., Brayard A., Bour I., Mathieu O., Elmeknassi S., Olivier N., Escarguel G., Bylund K. G., Jenks J., Stephen D. A., Fara E., A diagenetic control on the Early Triassic Smithian–Spathian carbon isotopic excursions recorded in the marine settings of the Thaynes Group (Utah, USA). Geobiology 14, 220–236 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Lucas S. G., Krainer K., Milner A. R. C., The type section and age of the Timpoweap Member and stratigraphic nomenclature of the Triassic Moenkopi Group in southwestern Utah. New Mexico Mus. Nat. Hist. Sci. Bull. 40, 109–117 (2007). [Google Scholar]
- 20.Brayard A., Bylund K., Jenks J., Stephen D., Olivier N., Escarguel G., Fara E., Vennin E., Smithian ammonoid faunas from Utah: Implications for Early Triassic biostratigraphy, correlation and basinal paleogeography. Swiss J. Paleontol. 132, 141–219 (2013). [Google Scholar]
- 21.S. Q. Dornbos, in Taphonomy: Process and Bias through Time, P. A. Allison, D. J. Bottjer, Eds. (Springer, 2010), pp. 435–456. [Google Scholar]
- 22.Castellani C., Maas A., Haug C., Haug J. T., Waloszek D., Isolated sponge spicules from the late Cambrian Alum Shale Formation (‘Orten’ nodules) of Sweden. Bull. Geosci. 87, 443–460 (2012). [Google Scholar]
- 23.Rigby J. K., Gosney T. C., First reported Triassic lyssakid sponges from North America. J. Paleontol. 57, 787–796 (1983). [Google Scholar]
- 24.Pisera A., Rigby J. K. Sr., Bylund K. G., Lower Triassic hexactinellid sponges from the Confusion Range, western Utah. Brigham Young Univ. Geol. Stud. 41, 139–148 (1996). [Google Scholar]
- 25.Brayard A., Vennin E., Olivier N., Bylund K. G., Jenks J., Stephen D. A., Bucher H., Hofmann R., Goudemand N., Escarguel G., Transient metazoan reefs in the aftermath of the end-Permian mass extinction. Nat. Geosci. 4, 693–697 (2011). [Google Scholar]
- 26.Marenco P. J., Griffin J. M., Fraiser M. L., Clapham M. E., Paleoecology and geochemistry of Early Triassic (Spathian) microbial mounds and implications for anoxia following the end-Permian mass extinction. Geology 40, 715–718 (2012). [Google Scholar]
- 27.Vennin E., Olivier N., Brayard A., Bour I., Thomazo C., Escarguel G., Fara E., Bylund K. G., Jenks J. F., Stephen D. A., Hofmann R., Microbial deposits in the aftermath of the end-Permian mass extinction: A diverging case from the Mineral Mountains (Utah, USA). Sedimentology 62, 753–792 (2015). [Google Scholar]
- 28.Olivier N., Brayard A., Vennin E., Escarguel G., Fara E., Bylund K. G., Jenks J. F., Caravaca G., Stephen D. A., Evolution of depositional settings in the Torrey area during the Smithian (Early Triassic, Utah, USA) and their significance for the biotic recovery. Geol. J. 51, 600–626 (2016). [Google Scholar]
- 29.Weaver J. C., Aizenberg J., Fantner G. E., Kisailus D., Woesz A., Allen P., Fields K., Porter M. J., Zok F. W., Hansma P. K., Fratzl P., Morse D. E., Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum. J. Struct. Biol. 158, 93–106 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Botting J. P., Muir L. A., Lin J.-P., Relationships of the Cambrian Protomonaxonida (Porifera). Palaeontol. Electron. 16, 9A (2013). [Google Scholar]
- 31.Muir L. A., Botting J. P., Carrera M. G., Beresi M., Cambrian, Ordovician and Silurian non-stromatoporoid Porifera. Geol. Soc. London Mem. 38, 81–95 (2013). [Google Scholar]
- 32.Botting J. P., Diversity and ecology of sponges in the Early Ordovician Fezouata Biota. Palaeogeogr. Palaeoclimatol. Palaeoecol. 460, 75–86 (2016). [Google Scholar]
- 33.Stanley G. D., Jr, A Triassic sponge from Vancouver Island: Possible holdover from the Cambrian. Can. J. Earth Sci. 35, 1037–1043 (1998). [Google Scholar]
- 34.Conway Morris S., Whittington H. B., Fossils of the Burgess Shale: A national treasure in Yoho National Park, British Columbia. Misc. Rep. Geol. Surv. Can. 43, 1–31 (1985). [Google Scholar]
- 35.Wang H., Zhang Z., Holmer L. E., Hu S., Wang X., Li G., Peduncular attached secondary tiering acrotretoid brachiopods from the Chengjiang fauna: Implications for the ecological expansion of brachiopods during the Cambrian explosion. Palaeogeogr. Palaeoclimatol. Palaeoecol. 323–325, 60–67 (2012). [Google Scholar]
- 36.Hagdorn H., Triassic: The crucial period of post-Palaeozoic crinoid diversification. Swiss J. Paleontol. 130, 91–112 (2011). [Google Scholar]
- 37.Oji T., Twitchett R. J., The oldest post-Palaeozoic crinoid and Permian–Triassic origins of the Articulata (Echinodermata). Zoolog. Sci. 32, 211–215 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Twitchett R. J., Feinberg J. M., O’Connor D. D., Alvarez W., McCollum L. B., Early Triassic ophiuroids: Their paleoecology, taphonomy, and distribution. Palaios 20, 213–223 (2005). [Google Scholar]
- 39.Baucon A., Neto de Carvalho C., Stars of the aftermath: Asteriacites beds from the Lower Triassic of the Carnic Alps (Werfen Frmation, Sauris di Sopra), Italy. Palaios 31, 161–176 (2016). [Google Scholar]
- 40.O’Hara T. D., Hugall A. F., Thuy B., Moussalli A., Phylogenomic resolution of the class Ophiuroidea unlocks a global microfossil record. Curr. Biol. 24, 1874–1879 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Thuy B., Stöhr S., A new morphological phylogeny of the Ophiuroidea (Echinodermata) accords with molecular evidence and renders microfossils accessible for cladistics. PLOS ONE 11, e0156140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs D., Larson N., Diversity, morphology, and phylogeny of coleoid cephalopods from the Upper Cretaceous plattenkalks of Lebanon—Part I: Prototeuthidina. J. Paleontol. 85, 234–249 (2011). [Google Scholar]
- 43.Doguzhaeva L. A., Mapes R. H., Arm hooks and structural features in the Early Permian Glochinomorpha Gordon 1971, indicative of its coleoid affiliation. Lethaia 48, 100–114 (2015). [Google Scholar]
- 44.Fuchs D., Iba Y., The gladiuses in coleoid cephalopods: Homology, parallelism, or convergence? Swiss J. Paleontol. 134, 187–197 (2015). [Google Scholar]
- 45.Buchy M.-C., Taugourdeau P., Janvier P., Stomach contents of a Lower Triassic ichthyosaur from Spitzbergen. Oryctos 5, 47–55 (2004). [Google Scholar]
- 46.Ehiro M., Sasaki O., Kano H., Nemoto J., Kato H., Thylacocephala (Arthropoda) from the Lower Triassic of the South Kitakami Belt, Northeast Japan. Pal. Res. 19, 269–282 (2015). [Google Scholar]
- 47.Ovtcharova M., Bucher H., Schaltegger U., Galfetti T., Brayard A., Guex J., New Early to Middle Triassic U-Pb ages from South China: Calibration with ammonoid biochronozones and implications for the timing of the Triassic biotic recovery. Earth Planet. Sci. Lett. 243, 463–475 (2006). [Google Scholar]
- 48.Ovtcharova M., Goudemand N., Hammer Ø., Guodun K., Cordey F., Galfetti T., Schaltegger U., Bucher H., Developing a strategy for accurate definition of a geological boundary through radio-isotopic and biochronological dating: The Early–Middle Triassic boundary (South China). Earth-Sci. Rev. 146, 65–76 (2015). [Google Scholar]
- 49.Burgess S. D., Bowring S., Shen S.-z., High-precision timeline for Earth’s most severe extinction. Proc. Natl. Acad. Sci. U.S.A. 111, 3316–3321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galfetti T., Bucher H., Martini R., Hochuli P. A., Weissert H., Crasquin-Soleau S., Brayard A., Goudemand N., Brühwiler T., Guodun K., Evolution of Early Triassic outer platform paleoenvironments in the Nanpanjiang Basin (South China) and their significance for the biotic recovery. Sediment. Geol. 204, 36–60 (2008). [Google Scholar]
- 51.Ware D., Bucher H., Brayard A., Schneebeli-Hermann E., Brühwiler T., High-resolution biochronology and diversity dynamics of the Early Triassic ammonoid recovery: The Dienerian faunas of the Northern Indian Margin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 440, 363–373 (2015). [Google Scholar]
- 52.Hermann E., Hochuli P. A., Méhay S., Bucher H., Brühwiler T., Ware D., Hautmann M., Roohi G., ur-Rehman K., Yaseen A., Organic matter and palaeoenvironmental signals during the Early Triassic biotic recovery: The Salt Range and Surghar Range records. Sediment. Geol. 234, 19–41 (2011). [Google Scholar]
- 53.Brühwiler T., Bucher H., Brayard A., Goudemand N., High-resolution biochronology and diversity dynamics of the Early Triassic ammonoid recovery: The Smithian faunas of the Northern Indian Margin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 297, 491–501 (2010). [Google Scholar]
- 54.Guex J., Hungerbühler A., Jenks J., O’Dogherty L., Atudorei V., Taylor D. G., Bucher H., Bartolini A., Spathian (Lower Triassic) ammonoids from western USA (Idaho, California, Utah and Nevada). Mem. Geol. Lausanne 49, 1–81 (2010). [Google Scholar]
- 55.Brayard A., Brühwiler A., Bucher H., Jenks J., Guodunites, a low-palaeolatitude and trans-Panthalassic Smithian (Early Triassic) ammonoid genus. Palaeontology 52, 471–481 (2009). [Google Scholar]
- 56.J. F. Jenks, C. Monnet, M. Balini, A. Brayard, M. Meier, in Ammonoid Paleobiology: From Macroevolution to Paleogeography, C. Klug, D. Korn, K. De Baets, I. Kruta, R. H. Mapes, Eds. (Springer, 2015), pp. 329–388. [Google Scholar]
- 57.Romano C., Kogan I., Jenks J., Jerjen I., Brinkmann W., Saurichthys and other fossil fishes from the late Smithian (Early Triassic) of Bear Lake County (Idaho, USA), with a discussion of saurichthyid palaeogeography and evolution. Bull. Geosci. 87, 543–570 (2012). [Google Scholar]
- 58.Massare J. A., Callaway J. M., Cymbospondylus (Ichthyosauria: Shastasauridae) from the Lower Triassic Thaynes Formation of southeastern Idaho. J. Vert. Paleontol. 14, 139–141 (1994). [Google Scholar]
- 59.Tosk T. A., Andersson K. A., Late Early Triassic foraminifers from possible dysaerobic to anaerobic paleoenvironments of the Thaynes Formation, Southeast Idaho. J. Foraminiferal Res. 18, 286–301 (1988). [Google Scholar]
- 60.Hofmann R., Hautmann M., Bucher H., A new paleoecological look at the Dinwoody Formation (Lower Triassic, western USA): Intrinsic versus extrinsic controls on ecosystem recovery after the end-Permian mass extinction. J. Paleontol. 87, 854–880 (2013). [Google Scholar]
- 61.Kummel B., The Thaynes formation, Bear Lake Valley, Idaho. Am. J. Sci. 241, 316–332 (1943). [Google Scholar]
- 62.Kummel B., Triassic stratigraphy of southeastern Idaho and adjacent areas. U.S. Geol. Surv. Prof. Paper 254, 165–194 (1954). [Google Scholar]
- 63.Jenks J., Guex J., Hungerbühler A., Taylor D., Bucher H., Ammonoid biostratigraphy of the Early Spathian Columbites parisianus Zone (Early Triassic) at Bear Lake Hot Springs, Idaho. New Mexico Mus. Nat. Hist. Sci. Bull. 61, 268–283 (2013). [Google Scholar]
- 64.Aigner T., Storm Depositional Systems. Dynamic Stratigraphy in Modern and Ancient Shallow-Marine Sequences. vol. 3 of Lecture Notes Earth Science (Springer, 1985), 174 pp. [Google Scholar]
- 65.G. Einsele, Sedimentary Basins. Evolution, Facies, and Sediment Budget (Springer, 1992). [Google Scholar]
- 66.A. Seilacher, T. Aigner, in Cycles and Events in Stratigraphy, G. Einsele, W. Ricken, A. Seilacher, Eds. (Springer, 1991), pp. 249–267. [Google Scholar]
- 67.Chen Z.-Q., Kaiho K., George A. D., Early Triassic recovery of the brachiopod faunas from the end-Permian mass extinction: A global review. Palaeogeogr. Palaeoclimatol. Palaeoecol. 224, 270–290 (2005). [Google Scholar]
- 68.Y. Shigeta, Y. D. Zakharov, H. Maeda, A. M. Popov, The Lower Triassic System in the Abrek Bay Area, South Primorye, Russia (National Museum of Nature and Science, Tokyo, 2009). [Google Scholar]
- 69.Spath L. F., Additions to the Eo-Triassic Invertebrate Fauna of East Greenland (C. A. Reitzel, Copenhagen, 1935), 115 pp. [Google Scholar]
- 70.B. Kummel, C. Teichert, Eds., Stratigraphic Boundary Problems: Permian and Triassic of West Pakistan (University Press of Kansas, 1970). [Google Scholar]
- 71.Fraiser M. L., Paleoecology of secondary tierers from Western Pangean tropical marine environments during the aftermath of the end-Permian mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 308, 181–189 (2011). [Google Scholar]
- 72.Ware D., Jenks J. F., Hautmann M., Bucher H., Dienerian (Early Triassic) ammonoids from the Candelaria Hills (Nevada, USA) and their significance for palaeobiogeography and palaeoceanography. Swiss J. Geosci. 104, 161–181 (2011). [Google Scholar]
- 73.Rigby J. K., Sponges of the Burgess Shale (Middle Cambrian), British Columbia. Palaeontogr. Can. 2, 1–105 (1986). [Google Scholar]
- 74.Holmer L. E., Middle Ordovician phosphatic inarticulate brachiopods from Vastergotland and Dalarna, Sweden. Fossils Strata 26, 1–172 (1989). [Google Scholar]
- 75.Lenz A. C., A Silurian sponge-inarticulate brachiopod life? association. J. Paleontol. 67, 138–139 (1993). [Google Scholar]
- 76.Holmer L. E., Popov L. E., Sreng M., Miller J. F., Lower Ordovician (Tremadocian) lingulate brachiopods from the House and Fillmore formations, Ibex area, western Utah, USA. J. Paleontol. 79, 884–906 (2005). [Google Scholar]
- 77.Wasmer M., Hautmann M., Hermann E., Ware D., Roohi G., Ur-Rehman K., Yaseen A., Bucher H., Olenekian (Early Triassic) bivalves from the Salt Range and Surghar Range, Pakistan. Palaeontology 55, 1043–1073 (2012). [Google Scholar]
- 78.Kummel B., Paleoecology of Lower Triassic formations of southeastern Idaho and adjacent areas. Geol. Soc. Am. Mem. 67, 437–468 (1957). [Google Scholar]
- 79.Jenks J., Spielmann J. A., Lucas S. G., Triassic ammonoids: A photographic journey. New Mexico Mus. Nat. Hist. Sci. Bull. 40, 33–79 (2007). [Google Scholar]
- 80.T. S. Engeser, M. R. Clarke, in Paleontology and Neontology of Cephalopods, E. R. Truman, M. R. Clarke, Eds. (Academic Press, 1988), pp. 133–151. [Google Scholar]
- 81.Garassino A., Donovan D. T., A new family of coleoids from the Lower Jurassic of Osteno, Northern Italy. Palaeontology 43, 1019–1038 (2000). [Google Scholar]
- 82.Stevens G. R., Palaeobiological and morphological aspects of Jurassic Onychites (cephalopod hooks) and new records from the New Zealand Jurassic. New Zeal. J. Geol. Geophys. 53, 395–412 (2010). [Google Scholar]
- 83.Rieber H., Phragmoteuthis? ticinensis n. sp., ein Coleoidea-Rest aus der Grenzbitumenzone (Mittlere Trias) des Monte San Giorgio (Kt. Tessin, Schweiz). Paläont. Z. 44, 32–40 (1970). [Google Scholar]
- 84.Motani R., Chen X.-h., Jiang D.-y., Cheng L., Tintori A., Rieppel O., Lunge feeding in early marine reptiles and fast evolution of marine tetrapod feeding guilds. Sci. Rep. 5, 8900 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doguzhaeva L. A., Summesberger H., Mutvei H., Brandstaetter F., The mantle, ink sac, ink, arm hooks and soft body debris associated with the shells in Late Triassic coleoid cephalopod Phragmoteuthis from the Austrian Alps. Palaeoworld 16, 272–284 (2007). [Google Scholar]
- 86.Doguzhaeva L. A., Mutvei H., Summesberger H., Dunca H., Bituminous soft body tissues in Late Triassic ceratitid Austrotrachyceras. Mitt. Geol-Paläontol. Inst. Univ. Hamburg 88, 37–50 (2004). [Google Scholar]
- 87.Schweigert G., Fuchs D., First record of a true coleoid cephalopod from the Germanic Triassic (Ladinian). Neues Jahrb. Geol. Paläontol. 266, 19–30 (2012). [Google Scholar]
- 88.Fuchs D., Octobrachia—A diphyletic taxon? Berliner Paläobiol. Abh. 10, 181–192 (2009). [Google Scholar]
- 89.Jattiot R., Brayard A., Fara E., Charbonnier S., Gladius-bearing coleoids from the Upper Cretaceous Lebanese Lagerstätten: Diversity, morphology, and phylogenetic implications. J. Paleontol. 89, 148–167 (2015). [Google Scholar]
- 90.Fuchs D., Weiss R., Taxonomy, morphology and phylogeny of Lower Jurassic teudopseid coleoids (Cephalopoda). Neues Jahrb. Geol. Paläontol. 257, 351–366 (2010). [Google Scholar]
- 91.Fuchs D., Weiss R., Teudopsis bunelii Eudes-Deslongchamps, 1835 (Cephalopoda: Coleoidea) from Upper Toarcian ironstones of Luxembourg. Ferrantia 62, 63–71 (2010). [Google Scholar]
- 92.Rieber H., Cephalopoden aus der Grenzbitnmenzone (Mittlere Trias) des Monte San Giorgio (Kanton Tessin, Schweiz). Schweiz Palaeont. Abh. 93, 1–96 (1973). [Google Scholar]
- 93.Rieber H., Breviconoteuthis breviconus (Reis), ein Phragmoteuthide aus der MittleIen Trias des Monte San Giorgio (Kanton Tessin, Schweiz). Neues J. Geol. Paläontol. Monat. 7, 415–421 (1974). [Google Scholar]
- 94.Iba Y., Sano S.-i., Mutterlose J., Kondo Y., Belemnites originated in the Triassic—A new look at an old group. Geology 40, 911–914 (2012). [Google Scholar]
- 95.Doguzhaeva L. A., Summesberger H., Pro-ostraca of Triassic belemnoids (Cephalopoda) from Northern Calcareous Alps, with observations on their mode of preservation in an environment of northern Tethys which allowed for carbonization of non-biomineralized structures. Neues Jahrbuch. Geol. Paläont. Abh. 266, 31–38 (2012). [Google Scholar]
- 96.Rosenkrantz A., Krogbærende cephalopoder fra Østgrønlands Perm. Medd. Dansk. Geol. Foren. 11, 160–161 (1946). [Google Scholar]
- 97.S. Charbonnier, A. Garassino, G. Schweigert, M. Simpson, A Worldwide Review of Fossil and Extant Glypheid and Litogastrid Lobsters (Crustacea, Decapoda, Glypheoidea) (Mémoires du Museum national d’Histoire naturelle, Paris, 2013), 307 pp. [Google Scholar]
- 98.Schram F. R., Litogaster turnbullensis (sp. nov.): A Lower Triassic glypheid decapod crustacean from Idaho. J. Paleontol. 45, 534–537 (1971). [Google Scholar]
- 99.Broda K., Hegna T. A., Zatoń M., Thylacocephalans. Geol. Today 31, 116–120 (2015). [Google Scholar]
- 100.Haug C., Briggs D. E. G., Mikulic D. G., Kluessendorf J., Haug J. T., The implications of a Silurian and other thylacocephalan crustaceans for the functional morphology and systematic affinities of the group. BMC Evol. Biol. 14, 159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schram F. R., Family level classification within Thylacocephala, with comments on their evolution and possible relationships. Crustaceana 87, 340–363 (2014). [Google Scholar]
- 102.Pinna G., Arduini P., Pesarini C., Teruzzi G., Some controversial aspects of the morphology and anatomy of Ostenocaris cypriformis (Crustacea, Thylacocephala). Earth Environ. Sci. Trans. R. Soc. Edinburgh 76, 373–379 (1985). [Google Scholar]
- 103.Secrétan S., Conchyliocarida, a class of fossil crustaceans: Relationships to Malacostraca and postulated behaviour. Earth Environ. Sci. Trans. R. Soc. Edinburgh 76, 381–389 (1985). [Google Scholar]
- 104.Vannier J., Chen J.-Y., Huang D.-Y., Charbonnier S., Wang X.-Q., The Early Cambrian origin of thylacocephalan arthropods. Acta Palaeontol. Polonica 51, 201–214 (2006). [Google Scholar]
- 105.S. Charbonnier, Le Lagerstätte de la Voulte : Un Environnement Bathyal au Jurassique (Mémoires du Muséum national d’Histoire naturelle, Paris, 2009). [Google Scholar]
- 106.Vannier J., Schoenemann B., Gillot T., Charbonnier S., Clarkson E., Exceptional preservation of eye structure in arthropod visual predators from the Middle Jurassic. Nat. Commun. 7, 10320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arduini P., Studies on Permo-Trias of Madagascar. 1. Thylacocephala from lower Trias of Madagascar. Atti. Soc. Ital. Sci. Nat. Museo Civico Storia Nat. Milano 131, 197–204 (1990). [Google Scholar]
- 108.Haig D. W., Martin S. K., Mory A. J., McLoughlin S., Backhouse J., Berrell R. W., Kear B. P., Hall R., Foster C. B., Shi G. R., Bevana J. C., Early Triassic (early Olenekian) life in the interior of East Gondwana: Mixed marine–terrestrial biota from the Kockatea Shale, Western Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 417, 511–533 (2015). [Google Scholar]
- 109.H. Hess, C. G. Messing, W. I. Ausich, Articulata, Part T: Echinodermata 2, vol. 3 of Crinoidea (University of Kansas Paleontological Institute, 2011). [Google Scholar]
- 110.Chen Z. Q., McNamara K. J., End-Permian extinction and subsequent recovery of the Ophiuroidea (Echinodermata). Palaeogeogr. Palaeoclimatol. Palaeoecol. 236, 321–344 (2006). [Google Scholar]
- 111.M. Ginter, O. Hampe, C. J. Duffin, Handbook of Paleoichthyology, Chondrichthyes Paleozoic Elasmobranchii: Teeth (Verlag Dr. Friedrich Pfeil, 2010). [Google Scholar]
- 112.H. Cappetta, Handbook of Paleoichthyology: Chondrichthyes, Mesozoic and Cenozoic Elasmobranchii: Teeth (Verlag Dr. Friedrich Pfeil, 2012). [Google Scholar]
- 113.Nielsen E., A preliminary note on Bobasatrania groenlandica. Medd. Dansk Geol. Forening 12, 197–204 (1952). [Google Scholar]
- 114.Böttcher R., Phyllodont tooth plates of Bobasatrania scutata (Gervais, 1852) (Actinoperygii, Bobasatraniiformes) from the Middle Triassic (Longobardian) Grenzbonebed of southern Germany and eastern France, with an overview of Triassic and Palaeozoic phyllodont tooth plates. Neues Jahrb. Geol. Paläontol. 274, 291–311 (2014). [Google Scholar]
- 115.Romano C., Ware D., Brühwiler T., Bucher H., Brinkmann W., Marine Early Triassic Osteichthyes from Spiti, Indian Himalayas. Swiss J. Palaeontol. 135, 275–294 (2016). [Google Scholar]
- 116.Evans H. M., A new cestraciont spine from the Lower Triassic of Idaho. Univ. Calif. Publ. Bull. Dep. Geol. 3, 397–402 (1904). [Google Scholar]
- 117.Goddard M., Fish remains from the marine lower Triassic of Aspen Ridge, Idaho. Univ. Calif. Publ. Bull. Dep. Geol. 5, 145–148 (1907). [Google Scholar]
- 118.Mutter R. J., Rieber H., Pyknotylacanthus spathianus gen. et sp. nov., a new ctenacanthoid from the Early Triassic of Bear Lake (Idaho, USA). Rev. Brasil. Paleontol. 8, 139–148 (2005). [Google Scholar]
- 119.Tanner V. M., A study of Utah fossil fishes with the description of a new genus and species. Proc. Utah Acad. Sci. Arts Lett. 13, 81–89 (1936). [Google Scholar]
- 120.Case E. C., A nothosaur from the Triassic of Wyoming. Univ. Michigan Contrib. Mus. Paleontol. 5, 1–36 (1936). [Google Scholar]
- 121.Northwood C., Early Triassic coprolites from Australia and their palaeobiological significance. Palaeontology 48, 49–68 (2005). [Google Scholar]
- 122.Hunt A. P., Lucas S. G., Spielmann J. A., Lerner A. J., A review of vertebrate coprolites of the Triassic with descriptions of new Mesozoic ichnotaxa. New Mexico Mus. Nat. Hist. Sci. Bull. 41, 88–107 (2007). [Google Scholar]
- 123.Yates A. M., Neumann F. H., Hancox P. J., The earliest post-Paleozoic freshwater bivalves preserved in coprolites from the Karoo Basin, South Africa. PLOS ONE 7, e30228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.F.-J. Lindemann, in Boreal Triassic 2006, H. A. Nakrem, A. Mørk, Eds. (Norsk Geologisk Forening, Abstracts and Proceedings of the Geological Society of Norway, 2006), pp. 94–96. [Google Scholar]
- 125.Nakajima Y., Izumi K., Coprolites from the upper Osawa Formation (upper Spathian), northeastern Japan: Evidence for predation in a marine ecosystem 5 Myr after the end-Permian mass extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 414, 225–232 (2014). [Google Scholar]
- 126.Scheyer T. M., Romano C., Jenks J., Bucher H., Early Triassic marine biotic recovery: The predators’ perspective. PLOS ONE 9, e88987 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brachaniec T., Niedźwiedzki R., Surmik D., Krzykawski T., Szopa K., Gorzelak P., Salamon M. A., Coprolites of marine vertebrate predators from the Lower Triassic of southern Poland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 435, 118–126 (2015). [Google Scholar]
- 128.Hao T.-q., Ji C., Sun Z.-y., Jiang D.-y., Tintori A., The Early Triassic conodonts in coprolites from Cahohu, Anhui. J. Stratigraphy 39, 188–196 (2015). [Google Scholar]
- 129.Kelley N. P., Motani R., Embree P., Orchard M. J., A new Lower Triassic ichthyopterygian assemblage from Fossil Hill, Nevada. PeerJ 4, e1626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Payne J. L., Lehrmann D. J., Christensen S., Wei J., Knoll A. H., Environmental and biological controls on the initiation and growth of a Middle Triassic (Anisian) reef complex on the Great Bank of Guizhou, Guizhou Province, China. Palaios 21, 325–343 (2006). [Google Scholar]
- 131.Baud A., Brandner R., Donofrio D. A., The Sefid Kuh Limestone—A late lower Triassic carbonate ramp (Aghdarband, NE-Iran). Abh. Geol. Bundesanst 38, 111–123 (1991). [Google Scholar]
- 132.Wignall P. B., Twitchett R. J., Permian–Triassic sedimentology of Jameson Land, East Greenland: Incised submarine channels in an anoxic basin. J. Geol. Soc. London 159, 691–703 (2002). [Google Scholar]
- 133.J. L. Payne, B. van de Schootbrugge, in Evolution of Primary Producers in the Sea, P. Falkowski, A. H. Knoll, Eds. (Academic Press, 2007) pp. 165–189. [Google Scholar]
- 134.Hunt S., Nixon M., A comparative study of protein composition in the chitin-protein complexes of the beak, pen, sucker disc, radula and oesophageal cuticle of cephalopods. Comp. Biochem. Physiol. 68, 535–546 (1981). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/2/e1602159/DC1
Supplementary Text
fig. S1. Field view of the upper part of the Lower Shale unit containing the Paris Biota.
fig. S2. Log section of the main Paris Biota exposure.
fig. S3. Taxa of the Paris Biota showing unexpected or extended temporal distributions, new Early Triassic spatiotemporal occurrences, new oldest records of derived characters for the clade, and new ecologic or trophic interactions.
fig. S4. Artistic reconstruction of the Paris Biota.
fig. S5. Closeup view of an apex of a leptomitid protomonaxonid sponge.
fig. S6. Closeup views of twisted apex of two leptomitid protomonaxonid sponges.
fig. S7. Closeup views of longitudinal and transverse spicules of four leptomitid protomonaxonid sponges.
figs. S8 to S10. Leptomitid protomonaxonid sponges from the Paris Biota.
fig. S11. Leptomitid protomonaxonid sponge from the Paris Biota showing attachment to a shell fragment.
fig. S12. Linguloid and discinoid inarticulate brachiopods.
fig. S13. Sponge specimen showing minute epizoan brachiopods and closeup views of epizoan brachiopods.
fig. S14. Bivalve specimens.
fig. S15. Ammonoid and nautiloid specimens.
fig. S16. Belemnoid arm hooks.
fig. S17. Gladius-bearing coleoid.
fig. S18. Different types of preservation for crustaceans from the Paris Biota.
fig. S19. Penaeidean and caridean shrimps and accumulations.
fig. S20. Penaeid shrimps, caridean shrimp, and litogastrid lobsters.
fig. S21. Thylacocephalan specimens.
fig. S22. Holocrinid specimens.
fig. S23. Closeup view of a crinoid stem with cirri and closeup view of an isolated pinnulate arm.
fig. S24. Ophiuroid specimens.
fig. S25. Closeup view of the spine articulations of an ophiuroid specimen.
fig. S26. Vertebrate remains from the Paris Biota.
fig. S27. Coprolites from the Paris Biota.
fig. S28. Algal morphotypes from the Paris Biota.
fig. S29 and S30. SEM photographs and EDS analyses for leptomitid protomonaxonid sponge specimens.
fig. S31. SEM photograph and EDS analysis for a crustacean specimen.
fig. S32. SEM photograph and EDS analysis for a belemnoid hook specimen.
fig. S33. SEM photograph and EDS analyses for a leptomitid protomonaxonid sponge specimen.
table S1. Sampled taxa from the Paris Biota.



