Abstract
Generally Early Triassic floras are believed to be depauperate, suffering from protracted recovery following the Permian–Triassic extinction event. Here we present palynological data of an expanded East Greenland section documenting recovered floras in the basal Triassic (Griesbachian) and a subsequent fundamental floral turnover, postdating the Permian–Triassic boundary extinction by about 500 kyrs. This event is marked by a swap in dominating floral elements, changing from gymnosperm pollen-dominated associations in the Griesbachian to lycopsid spore-dominated assemblages in the Dienerian. This turnover coincides with an extreme δ13Corg negative shift revealing a severe environmental crisis, probably induced by volcanic outbursts of the Siberian Traps, accompanied by a climatic turnover, changing from cool and dry in the Griesbachian to hot and humid in the Dienerian. Estimates of sedimentation rates suggest that this environmental alteration took place within some 1000 years. Similar, coeval changes documented on the North Indian Margin (Pakistan) and the Bowen Basin (Australia) indicate the global extent of this crisis. Our results evidence the first profound disruption of the recovery of terrestrial environments about 500kyrs after the Permian–Triassic extinction event. It was followed by another crisis, about 1myrs later thus, the Early Triassic can be characterised as a time of successive environmental crises.
Scientists as well as the broad public become more and more aware of modern (manmade) destruction of terrestrial environments caused not only by the obvious effects of air pollution or clearing rain forests but also by the more subtle and long-term climatic change1,2. Deforestation is associated with the loss of biodiversity, increased greenhouse gas emissions, disrupted water cycles, increased soil erosion, and disrupted lebensraum3,4,5,6,7. These effects are also regularly discussed as consequences of the events leading to the Phanerozoic mass extinctions8. In fact there is increasing evidence that we are currently witnessing the sixth–manmade-mass extinction, associated with the destruction of natural environments and climatic change9. Thus, the hypothesis published a few years ago that the Permian–Triassic mass extinction (PTME), the biggest extinction event in Earth history (ca. 251myrs ago), was associated with an essentially global deforestation10,11 was easily accepted by the scientific community. This led to the widely approved concept that the destruction of the gymnosperm forests induced a pioneer vegetation of herbaceous lycopsids that subsequently dominated the terrestrial environments during the entire Early Triassic. Furthermore, the climate of the Early Triassic was interpreted as homogeneously warm and semi-arid12 or hot and arid13,14. These ideas concurred with the commonly hawked hypothesis that Early Triassic biotas reflect a protracted recovery after the PTME. Recent studies, however, show a more complex picture of the development of the biosphere and climate during this time, providing evidence for significant climatic changes15 as well as for profound faunal and floral turnovers16,17,18,19,20,21,22,23,24,25. New evidence suggest that not all marine organisms were similarly affected by the PTME. Most linages of conodonts and numerous radiolarians survived this event, but went extinct around the Griesbachian–Dienerian boundary while typical Mesozoic conodont and radiolarian assemblages appeared after this event17,18. Other Griesbachian records have reported “incredibly diverse benthic fauna”19, and “unexpectedly diverse and complex ichnofauna”20, which reveal at least locally sound living conditions for benthic ecosystems. Hence, these findings suggest that during the Griesbachian environmental conditions were less hostile than generally assumed and that an important extinction event affecting several groups occurred around the Griesbachian–Dienerian boundary17,18,20,23. Palynological studies of expanded sections with well-preserved pollen and spores have provided insight into the dynamics of the development of plant associations during these critical intervals. New results show that the PTME lead to short term changes in the plant community abundance structure, expressed in the palynological records by a “spore spike”10,24,26. Thus, the immediate reaction of the plant communities on the environmental catastrophe of the PTME was similar to those known from the subsequent major cataclysms, such as at the Triassic–Jurassic boundary (TJB) or the Cretaceous-Paleogene events (KPE), which are also characterized by short-lived super-abundance of pteridophyte spores27,28,29,30. These “spore spikes” systematically concur with negative δ13C isotope shifts. Thus, they obviously reflect reaction of the vegetation to environmental cataclysms, such as massive volcanic outbursts related to the PTME or the TJB28,31,32 or to the asteroid impact at the KPB33. This shows that different causes of disruption of terrestrial ecosystems lead to a similar reactions of the vegetation, even concerning various groups of pteridophytes–lycopsids at the PTME versus ferns at the TJB and the KPB. In fact in modern disturbed ecosystems ferns and fern allies play an important role in ecosystem restoration due to their tolerance to grow in acidic water-logged conditions or their ability to exploit nutrient-poor substrates–conditions that are associated with volcanic outbursts29,34,35. After the clearance of primary forests these opportunistic plants are known to take over and give subsequently way to the succeeding forests34,36. High resolution palynological data including the PTME indicate that gymnosperms recovered probably within a few kyrs to become again the dominating floral element24. Some typical Permian floral elements (e.g., Vittatina) gradually disappeared during this phase of recovery, although without an obvious loss in biodiversity or extinction of major plant groups24,37. Instead the detailed records from the Finnmark Platform24,37 show a distinct diversification of both gymnosperms and pteridophytes, which results in increased diversities after the PTME. New radiometric datings of the Permian–Triassic succession of the Bowen Basin38 shed a new light on the impact of PTME on plants. According to this new calibration the Playfordiaspora crenulata–and the Protohaploxypinus microcorpus zones, formerly attributed to the Late Permian, are now dated as Induan. Consequently, the floral turnover between the two zones described by Foster39 reflects now a mid-Induan event. The palynological assemblages of the P. crenulata zone are characterised by high abundance and diversity of taeniate bisaccate pollen changing at the boundary to the P. microcorpus zone to a dominance of lycopsid spores. Above all this event is marked by the disappearance of the Glossopteris flora39.
The Griesbachian record from East Greenland and the coeval section in the Trøndelag area of mid-Norway show well diversified spore-pollen assemblages, with clear dominance of gymnosperms40. This evidence is in obvious contrast with the previous general conception of depauperate Early Triassic sporomorph assemblages that are assumed to be characterized by overall dominance of lycopsid spores12. The present study of the expanded Kap Stosch section (Hold with Hope, East Greenland, 73°60′N/21°12′W–74°04′N/21°43′W) reveals that the profound and sustained floral change leading to the dominance of lycopsids happened ca. 500 kyrs after the PTME21,41,42,43 around the Griesbachian–Dienerian boundary (GDB). Here the gymnosperm pollen dominated floral associations of the basal Triassic (Griesbachian) changed to lycopsid spore-dominated assemblages. Evidence from other areas (e.g., Barents Sea, Pakistan, Tibet) shows that lycopsid subsequently dominated the flora up into the middle Smithian, for about 1myrs where other floral changes of global extent led to a renewed and short lived dominance of gymnosperms in the late Smithian22,23,44. Late Early Triassic (Spathian) floras are characterised by mixed assemblages showing a general decline of lycopsid spores associated with renewed diversification of terrestrial floras towards the Middle Triassic (e.g., 23, 45, 46).
Results
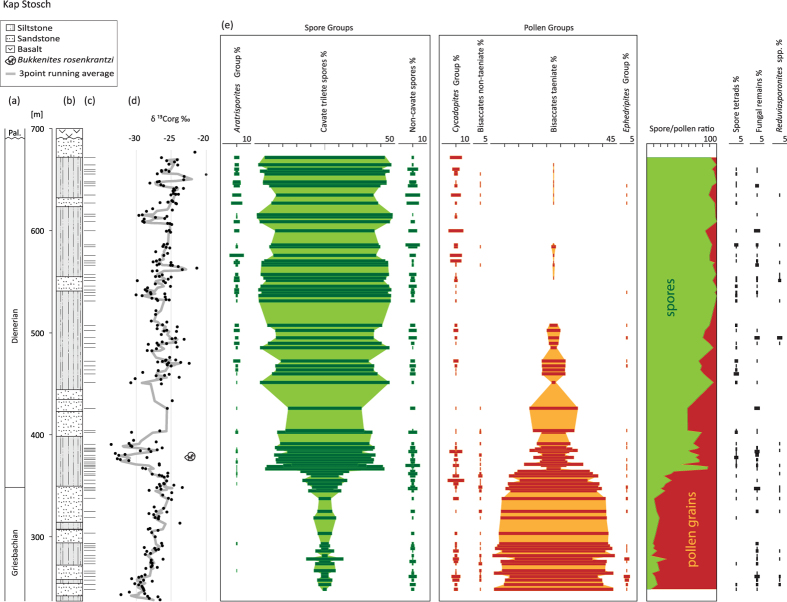
Here the general bulk palynological record with over 80 samples covering the upper part of the Griesbachian and the Dienerian from Kap Stosch is presented. The studied section is part of the Late Permian–Early Triassic Greenland-Norway rift basin. Deltaic sediments of the Wordie Creek Formation with changing marine influence were deposited during the latest Permian and the Induan (Griesbachian and Dienerian). The Griesbachian part of the formation comprises about 270m of section of which the upper 110 metres are included in the present study (Fig. 1). Age control is provided by a few horizons with ammonoids and bulk organic carbon isotope chemostratigraphy47,48. The Griesbachian–Dienerian boundary (GDB) is placed at the onset of a negative δ13Corg shift with the ammonoid Bukkenites rosenkrantzi occurring just above this level. Diverging from the interpretation of Bjerager et al.45 B. rosenkrantzi is considered here to be of Dienerian age because the worldwide oldest occurrences of proptychitids to which this species belongs are of Dienerian age or younger21.
Figure 1. Overview on Griesbachian–Dienerian floras with generalized C-isotope curve.
(a) Age (b) lithology (c) sample levels (d) bulk organic carbon isotopes46 (e) palynological data including relative abundances of groups of spore and pollen grains, spore/pollen ratios as well as spore tetrads, fungal remains and Reduviasporonites spp.
For the present study we differentiated seven main floral elements, which are mentioned below together with the essential changes in their distribution (Fig. 1). The first group–Aratrisporites-is generally associated with the macrofossils Annalepis, a probably herbaceous lycopsid of isoetalean affinity47. This group, being very rare in the Griesbachian, becomes a significant element in the upper part of the section. Cavate trilete spores, comprising essentially the genera Densoisporites, Kraeuselisporites, and Lundbladispora can be attributed to lycopsids, probably also to the Isoetales47. They show average abundances of 10–20% in the Griesbachian; increasing around the GDB (at ca. 350 m) to about 60%, to become the overwhelmingly dominant group with up to 90% of the assemblages in the uppermost part of the section (from ca. 450 m onward). Non-cavate spores comprise a variety of smooth and ornamented spores, which represent various pteridophytes (lycopsids and ferns) but also bryophytes. This group shows no significant abundance change within the record. Pollen grains summarized under Cycadopites group comprise a great variety of possible gymnosperm parent plants (e.g., Bennettiales, Ginkgoales, Cycadales, and Peltaspermales)47. They are relatively common in the lower part of the succession and up to 10% in some samples of the GDB interval. They represent essentially the only group of gymnosperms (up to 10%) in the uppermost part of the studied section. Non-taeniate bisaccate pollen grains include conifers but also some groups of pteridosperms. This group is rare in the upper part of the Griesbachian and almost disappears in the Dienerian. Taeniate bisaccate pollen grains, attributed to pteridosperms, represent the dominant group in the lower part of the record with abundances up to 90%. Around the GDB their abundance decreases gradually over a short interval from around 60% to about 20%, and they become rare (<5%) in the uppermost part of the section. The Ephedripites group, representing Gnetales47, is regularly observed in the lowermost part of the record with abundances up to 5% and occurs only sporadically in the upper part of the section. The spore/pollen ratio summarizes the essential changes in the plant assemblages. This ratio is regarded as a rough indicator for water availability to the plant communities24,48 Because they have played a significant role in the interpretation of the environmental disaster of the PTME11,49 the quantitative distribution of spore tetrads (comprising essentially Densoisporites spp.), fungal remains, and Reduviasporonites spp. are also included. In the present record their abundances show no significant trend. Generally, the trends in the distribution of all the above mentioned groups are unrelated to changes in the depositional environment46.
In the Kap Stosch area the Induan (Griesbachian and Dienerian) interval comprises about 600 m of section46. Thus, accepting the roughly estimated duration of the Induan of about 1.0 myr41,42,43 would result in an average sedimentation rate of ca. 0.6 m/kyr in the studied section. The estimated duration of the Dienerian at 416 kyrs by Ware et al.21 would suggest a sedimentation rate of 0.79 m/kyr for the 328 m of Dienerian section, provided that the entire substage is represented. The described mid-Induan floral turnover takes place over an interval of a ca. 4 metres at the onset of the prominent δ13Corg shift close to the GDB, thus we conclude that the changes occurred over a very short interval in the order of a few kyrs.
Discussion
Compared to the marine fauna relatively little is known about the impacts of the PTME and following events on the terrestrial realm. The record of Early Triassic plant macrofossils is extremely poor and mostly uncalibrated; however, palynological records adequately reflect floral changes during mass extinctions and the subsequent recovery phases22,23,25. For the PTME some spectacular scenarios have been inferred from relatively few records, e.g., total extinction50 totally devastated environments unsuited for plants12,49,51,52, plant mutagenesis due to ozone layer destruction10, and collapse of terrestrial ecosystems related to a fungal event49,52. Evidence for the latter event is still questionable, mainly because of the ambiguous biological attribution of Reduviasporonites, the key witness of this event, assigned by some authors to fungi53 and by others-with more convincing evidence-to algae54,55. However, high abundances of Reduviasporonites, as those interpreted as “fungal event”, have not been observed in any expanded section. Reduviasporonites occurs regularly but not abundantly throughout these records24,37,39.
Another effect of the PTME has been inferred from high numbers of spore tetrads in sediments comprising this event. It has been proposed that stratospheric ozone layer depletion and subsequent increased UV-B radiation caused genetic damage (mutagenesis) on lycopsids11. According to these authors the effect of this event prevented the spore tetrads of some lycopsids to separate during maturation. In fact peak abundances of tetrads coincide with the above mentioned “spore spike”. On the other hand, the tendency to shed the spores in tetrads could also be inherent to the lycopsid groups in question, as it is known for other fossil plants, such as the conifer pollen Triadispora or Classopollis. Other authors relate the high abundance of tetrads to depositional environments adjacent to the plant’s habitat56. Thus, the reasons for these occurrences remain ambiguous. In all expanded palynological records (Kap Stosch, Trøndelag and Finnmark platform) spore tetrads are regularly observed throughout the Griesbachian and the Dienerian. In the Kap Stosch record levels with increased numbers seem to be randomly distributed and are certainly unrelated to the GDB event (Fig. 1).
Due to the lack of adequate sections, preservational bias, or lack of stratigraphic calibration basal Early Triassic terrestrial successions are relatively poorly known. Numerous palynological studies of the Late Permian contain relatively rich assemblages whereas the overlying Triassic sections appear impoverished or consist of a few samples only12,55,57,58,59,60 and therefore emphasise the impression of an impoverished Early Triassic flora. A few well calibrated quantitative palynological records exist for the basal Triassic of the Barents Sea area as well as for the classical Early Triassic sections in the Salt Range and the Surghar Range in Pakistan, and based on recent radiometric age datings, for the Bowen Basin (eastern Australia)23,24,25,37,39,40. Calibration of the so far poorly constrained Australian Permian–Triassic palynostratigraphic succession shows that the most important floral turnover, associated with the extinction of the glossopterids, happened not at the Permian–Triassic boundary but within the Induan. Similar to the changes observed in E-Greenland this turnover is expressed by a strong reduction of the abundance and diversity of the pteridosperms (taeniate bisaccate pollen) and by a corresponding increase in lycopsids38,39.
The present record from East Greenland together with other high resolution terrestrial datasets challenges some of the running hypothesis associated with the PTME and its consequences for the Early Triassic flora. In contrast to the above mentioned spectacular extinction scenarios, the data from expanded latest Permian–earliest Triassic interval from the Southern Barents Sea (Finnmark platform) shows a distinct “spore spike” in reaction to the PTME37, i.e. reflecting not an extinction event, but a shift in the vegetation’s abundance structure. This event is also documented in a coeval section in East Greenland10,26. The data from the Finnmark platform24 and from E-Greenland10 suggest that this spore spike was of short duration–in the order of 10 kyrs. The succession following this “spore spike”, well documented in the Finnmark section, suggests a return to conditions similar to pre-event status, although with a gradual loss of some typical Late Permian elements during the Griesbachian24,37,40. The detailed study of this section shows the disappearance of several species–namely of the Vittatina group-near the PTB associated with numerous taxa appearing near this level7,61. The Griesbachian assemblages from Kap Stosch illustrate a rich and diverse flora subsequent to the PTME, similar to coeval records previously documented from the Finnmark and the Trøndelag platforms40. For eastern Australia Foster39 described the difference between the palynozone “Upper Stage 5”, now attributed to the Late Permian, and the basal Induan P. crenulata zone as gradual, marked only by the first occurrence of a few marker species. Thus the evidence for a sudden plant extinction related to the PTME becomes more and more scanty.
The presented floral record provides a so far unique insight on environmental changes during the aftermath of the PTME. Pronounced dominance of pteridosperms can be inferred from the lower part of the Induan records from the Northern hemisphere (Kap Stosch area, Finnmark and Trøndelag platforms) as well as from eastern Australia39. In the Kap Stosch section we can document a take-over of lycopsid spores of isoetalean affinity around the calibrated GDB. This turn-over is followed by a further decline of the gymnosperms during the Dienerian, ending in the top part of the section with an overall dominance of the lycopsids. The first decisive floral change coincides with the onset of a marked negative δ13Corg isotopic shift46. A subsequent change to the total dominance of spores in the middle part of the Dienerian concurs with the onset of another, although minor, negative δ13Corg shift (Fig. 1).
Comparable quantitative palynological records of Griesbachian and especially of Dienerian age from other areas are extremely rare. However, Schneebeli-Hermann et al.25 documented a coeval succession from the Salt Range (Pakistan). Although this section is strongly condensed (4m of section), it shows for the Griesbachian a strong dominance of gymnosperms-including pollen grains of glossopteridalean affinity, traditionally considered Permian markers, together with Triassic floral elements (e.g., Corystospermales, Dicroidium spp.)-followed by a gradual change to spore dominance in the Dienerian. In the Bowen Basin the significant decrease in the diversity and abundance of gymnosperms and the corresponding increase of lycopsids, coinciding with the disappearance of glossopterids, falls within the Induan, without further precision38,39. Detailed faunal data from the Salt Range sections reveals a low diversity ammonoid fauna at the base of the Dienerian, followed by a further diversity decrease in the middle Dienerian, and by a slight increase in the late Dienerian and finally by a significant diversification in the early Smithian21. Supposing that these ammonoid faunas reflect the general environmental quality, the middle Dienerian faunal assemblages would suggest most hostile conditions. The almost total demise of the gymnosperms in the Kap Stosch section might coincide with this faunal crisis and confirm a severe and prolonged global environmental crisis during the Dienerian.
Generally climatic changes are the primary trigger for changes in vegetation patterns. Fast reactions of the plant communities-within time spans of 100 yrs-are reliably recorded in changes in pollen assemblages (e.g., Early Holocene afforestation in the Alps62). In order to understand terrestrial environmental changes in the Early Triassic indicated by pronounced negative δ13Corg shifts and the coeval floral turnovers we try to infer the corresponding climatic conditions. Proliferation of spores is generally associated with more humid conditions, based on the fact that pteridophytes need liquid water at least during part of their life cycle. Thus spore dominated assemblages are considered to reflect relatively humid conditions. Temperature values are much more delicate to infer from plant records. Recent data of δ18O measurements of pristine biogenic apatite of conodonts reveal considerable changes in temperatures near the PTME and during the Early Triassic15. These authors inferred relatively cool temperatures for the Griesbachian and a temperature increase for the Dienerian. The δ18O record of the Early Triassic follows the trends in the carbon cycle reflected in the δ13C curve. The marked shifts in these records, reflecting severe environmental disturbances, are accompanied by major changes in plant assemblages (Fig. 2). For the Salt Range sections the increased spore ratios near the GDB and in the middle Smithian can be directly linked to negative shifts of δ13Corg and to lower δ18O15,23. These trends are interpreted to correspond to higher pCO2 and to higher temperatures that apparently induced at least seasonally increased humidity15. In contrast, the gymnosperms dominated assemblages of the late Smithian/early Spathian are associated with relatively positive δ13Corg values and δ18O values reflecting relatively cool temperatures15. Applying this relationship to the Induan succession of Kap Stosch, we suggest that the gymnosperm pollen dominated Griesbachian assemblages reflect relatively cool and dry conditions, which rapidly changed to hot and humid at the GDB.
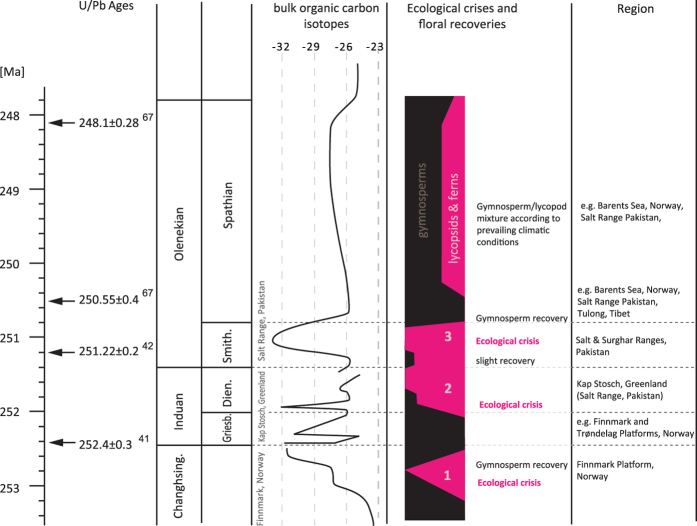
Figure 2. Summary of Early Triassic floral events.
Radiometric ages41,42,67 together with a simplified bulk organic carbon isotope curve24,48,50 are shown in relation to floral events documented in the Boreal Realm22,24,26,45 and on the North Indian margin23,25,44.
Similar to the GDB event the onset of the δ13C negative shift leading to the minimum near the PTB, coincides with a sudden increase of spores24. Similar relationships have been observed in the middle Smithian23; near the TJB28,32,63 and near the KPB64 where spore maxima are also associated with negative δ13C shifts. These changes reflect severe environmental and climatic changes, which in the case of the Early Triassic are probably associated with effects of successive volcanic outbursts related to the Siberian Large Igneous Province. Within the studied interval the dominance of lycopsids got stepwise accentuated, with increasing percentages of herbaceous forms, which become regular elements of the vegetation during the Dienerian. In contrast to the PTME, where the vegetation is interpreted to have recovered within a short time, the dominance of the lycopsids, appearing at the GDB, lasted–with a slight recovery in the early Smithian-for several 100 kyrs, up to the late Smithian23. Similar changes documented for the mid Induan of the North Indian Margin and eastern Australia suggest a global extent of this event. Thus, the GDB event, reflected in the floral turn-over associated with the extinction of the glossopterids, the pronounced negative δ13Corg shift, and the extinction in some marine groups (conodonts and radiolarians) is interpreted to represent another severe environmental disruption in a series of events such as at the PTB-, the mid-Smithian-, the TJB or the KPB. Recent progress in the technique of radiometric dating enables us to calibrate changes in continental sections and to put these deep-time environmental cataclysms within precise temporal frameworks and make them comparable to rapid changes that happened during the last 100kyrs. The floral turnovers related to the PTME and to the GDB event are thought to have happened within a few kyrs; thus, they become realistic models for future changes.
Methods
Sampling for palynology essentially focussed on fine grained, dark siltstones and mudstones; gaps in the record correspond to coarse clastic intervals. For the studied section 94 samples have been prepared following standard palynological preparation technique23 80 samples contain well preserved sporomorphs. For each sample a minimum of 250 sporomorphs has been counted. The detailed description of the section, palynofacies analysis, isotope measurements and palaeoenvironmental interpretations has been previously published by Sanson-Barrera et al.48.
Additional Information
How to cite this article: Hochuli, P. A. et al. Severest crisis overlooked—Worst disruption of terrestrial environments postdates the Permian–Triassic mass extinction. Sci. Rep. 6, 28372; doi: 10.1038/srep28372 (2016).
Acknowledgments
Maximiliano Meier is thanked for his assistance during field work. The project was supported by the Swiss NSF project 20021-135446/1 to HB. We acknowledge comments from two anonymous reviewers.
Footnotes
Author Contributions H.B. and P.A.H. designed the project; fieldwork has been carried out by H.B., P.A.H. and A.S.-B. Palynology analysis has been done by P.A.H., A.S.-B. and E.S.-H. and the write-up by P.A.H., E.S.-H., A.S.-B. and H.B.
References
- Cramer W. et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biology 7, 357–373 (2001). [Google Scholar]
- Vitousek P. M. Beyond global warming: Ecology and global change. Ecology 75, 1861–1876 (1994). [Google Scholar]
- Harper G. J., Steininger M. K., Tucker C. J., Juhn D. & Hawkins F. Fifty years of deforestation and forest fragmentation in Madagascar. Environmental Conservation 34, 325–333 (2007). [Google Scholar]
- Fearnside P. M. Deforestation in Brazilian Amazonia: History, Rates, and Consequences. Conservation Biology 19, 680–688 (2005). [Google Scholar]
- Werth D. & Avissar R. The local and global effects of Amazon deforestation. Journal of Geophysical Research 107, 8087, doi: 10.1029/2001JD000717 (2002). [DOI] [Google Scholar]
- Skole D. & Tucker C. Tropical deforestation and habitat fragmentation in the Amazon: Satelite Data from 1978 to 1988. Science 260, 1905–1910 (1993). [DOI] [PubMed] [Google Scholar]
- Zheng F. L. Effect of vegetation change on soil erosion on the Loess Plateau. Pedosphere 16, 420–427 (2006). [Google Scholar]
- Erwin D. H., Bowring S. A. & Jin Y. End-Permian mass extinctions: A review. Geological Society of America Special Paper 356, 363–383 (2002). [Google Scholar]
- Barnosky A. D. et al. Has the Earth’s sixth mass extinction already arrived? Nature 471, 51–57 (2011). [DOI] [PubMed] [Google Scholar]
- Looy C. V., Twitchett R. J., Dilcher D. L., van Konijnenburg-van Cittert J. H. A. & Visscher H. Life in the end-Permian dead zone. Proceedings of the National Academy of Sciences of the United States of America 98, 7879–7883 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher H. et al. Environmental mutagenesis during the end-Permian ecological crisis, Proceedings of the National Academy of Sciences of the United States of America 101, 12952–12956 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looy C. V., Brugman W. A., Dilcher D. L. & Visscher H. The delayed resurgence of equatorial forests after the Permian-Triassic ecologic crisis. Proceedings of the National Academy of Sciences of the United States of America 96, 13857–13862 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov N. M. & Zharkov M. A. Climate during the Permian-Triassic biosphere reorganization. Article 2. Climate of the Late Permian and Early Triassic: General inferences. Stratigraphy and Geological Correlation 11, 361–375 (2003). [Google Scholar]
- Preto N., Kustatscher E. & Wignall P. B. Triassic climate—State of the art and perspectives. Palaeogeography, Palaeoclimatology, Palaeoecology 290, 1–10 (2010). [Google Scholar]
- Romano C. et al. Climatic and biotic upheavals following the end-Permian mass extinction. Nature Geoscience 6, 57–60 (2013). [Google Scholar]
- Brühwiler T., Bucher H., Brayard A. & Goudemand N. High-resolution biochronology and diversity dynamics of the Early Triassic ammonoid recovery: The Smithian faunas of the Northern Indian Margin. Palaeogeography, Palaeoclimatology, Palaeoecology 297, 491–501 (2010). [Google Scholar]
- Orchard M. J. Conodont diversity and evolution through the latest Permian and Early Triassic upheavals. Palaeogeography, Palaeoclimatology, Palaeoecology 252, 93–117 (2007). [Google Scholar]
- O’Daugherty L., Carter E. S., Gorican S. & Dumitrica P. Triassic readiolarian biostratigraphy. Geological Society, London Special Publications 334, 163–200 (2010).
- Twitchett R. J., Krystyn L., Baud A., Wheeley J. R. & Richoz S. Rapid marinerecovery after the end-Permian mass-extinction event in the absence of marineanoxia. Geology 32, 805–808 (2004). [Google Scholar]
- Hofmann R., Goudemand N., Wasmer M., Bucher H. & Hautmann M. New trace fossil evidence for an early recovery signal in the aftermath of the end-Permian mass extinction. -Palaeogeography, Palaeoclimatology, Palaeoecology 310, 216–226 (2011). [Google Scholar]
- Ware D., Bucher H. Brayard A. Schneebeli-Hermann E. & Brühwiler T. High-resolution biochronology and diversity dynamics of the Early Triassic ammonoid recovery: The Dienerian faunas of the Northern Indian Margin. Palaeogeography, Palaeoclimatology, Palaeoecology 440, 363–373 (2015). [Google Scholar]
- Galfetti T. et al. Smithian–Spathian boundary event: Evidence for global climatic change in the wake of the end-Permian biotic crisis. Geology 35, 291–294 (2007). [Google Scholar]
- Hermann E. et al. Terrestrial ecosystems on North Gondwana following the end-Permian mass extinction. Gondwana Research 20, 630–637 (2011). [Google Scholar]
- Hochuli P. A., Hermann E., Vigran J. O., Bucher H. & Weissert H. Rapid demise and recovery of plant ecosystems across the end-Permian extinction event. Global and Planetary Change 74, 144–155 (2010). [Google Scholar]
- Schneebeli-Hermann E. et al. Vegetation history across the Permian–Triassic boundary in Pakistan (Amb section, Salt Range). Gondwana Research 24, 911–924 (2015). [Google Scholar]
- Stemmerik L., Bendix-Almgreen S. E. & Piasecki S. The Permian-Triassic boundary in central East Greenland: past and present views. Bulletin of the Geological Society of Denmark 48, 159–167 (2001). [Google Scholar]
- Tschudy R. H., Pillmore C. L., Orth C. J., Gilmore J. S. & Knight J. D. Disruption of the terrestrial Plant ecosystem at the Cretaceous-Tertiary boundary, Western Interior. Science 225, 1030–1032 (1984). [DOI] [PubMed] [Google Scholar]
- van de Schootbrugge B. et al. Floral changes across the Triassic/Jurassic boundary linked to flood basalt volcanism. Nature Geoscience 2, 589–594 (2009). [Google Scholar]
- Vajda V., Raine J. I. & Hollis C. J. Indication of global deforestation at the Cretaceous-Tertiary boundary by New Zealand fern spike. Science 294, 1700–1702 (2001). [DOI] [PubMed] [Google Scholar]
- Maruoka T., Koeberl C. & Bohor B. F. Carbon isotopic compositions of organic matter across continental Cretaceous–Tertiary (K–T) boundary sections: Implications for paleoenvironment after the K–T impact event. Earth and Planetary Science Letters 253, 226–238 (2007). [Google Scholar]
- Hesselbo S. P., Robinson S. A., Surlyk F. & Piasecki S. Terrestrial and marine extinction at the Triassic-Jurassic boundary synchronized with major carbon-cycle perturbation: A link to initiation of massive volcanism? Geology 30, 251–254 (2002). [Google Scholar]
- Bonis N. R., Ruhl M. & Kürscher W. M. Milankovitch-scale palynological turnover across the Triassic–Jurassic transition in St. Audrie’s Bay, SW UK. Journal of the Geological Society, London 167, 877–888 (2010). [Google Scholar]
- Alvarez. L. W., Alvarez W., Asaro F. & Michel H. V. Extraterrestrial cause for the Cretaceous–Tertiary extinction. Science 208, 1095–1108 (1980). [DOI] [PubMed] [Google Scholar]
- Walker L. R. & Sharpe J. M. Ferns, disturbance and succession in Fern ecology (eds Mehltreter K. Walker L. R. & Sharpe J. M.) 177–219 (Cambridge University Press, 2010). [Google Scholar]
- Page C. N. 2004. Adaptive ancientness of vascular plants to exploitation of low-nutrient substrates–a neobotanical overview in The evolution of plant Physiology (eds Hemsley R. A. & Poole I.) 446–466 (Elsevier Academic Press, 2004). [Google Scholar]
- Whittaker R. J., Bush M. B. & Richards K. Plant recolonization and vegetation succession on the Krakatau Islands, Indonesia. Ecological Monographs 59, 59–123 (1989). [Google Scholar]
- Mangerud G. Palynostratigraphy of the Permian and lowermost Triassic succession, Finnmark Platform, Barents Sea. Review of Palaeobotany and Palynology 82, 317–349 (1994). [Google Scholar]
- Nicoll R. et al. CA-ICTIMS dating of tuffs, calibration of palynostratigraphy and stratigraphy of the Bowen and Galilee basins. Bowen Basin Symposium 2015. 211–218 (2015).
- Foster C. B. Spore-pollen assemblages of the Bowen Basin, Queensland (Australia): Their relationship to the Permian/Triassic boundary, Review of Palaeobotany and Palynology 36, 165–183 (1982). [Google Scholar]
- Hochuli P. A., Vigran J. O., Hermann E. & Bucher H. Multiple climatic changes around the Permian–Triassic boundary event revealed by an expanded palynological record from mid-Norway. Geol. Soc. Am. Bull. 122, 884–896 (2010). [Google Scholar]
- Mundil R., Ludwig K. R., Metcalfe I. & Renne P. R. Age and timing of the Permian mass extinction: U/Pb dating of closed-system zircon. Science 305, 1760–1763 (2004). [DOI] [PubMed] [Google Scholar]
- Galfetti T. et al. Timing of the Early Triassic carbon cycle perturbations inferred from new U/Pb ages and ammonoid biochronozones. Earth and Planetary Science Letters 258, 593–604 (2007). [Google Scholar]
- Gradstein F. M., Ogg J. G., Schmitz M. D. & Ogg G. M. The geological time scale (Elsevier, 2012). [Google Scholar]
- Schneebeli-Hermann E. et al. Palynology of the Lower Triassic succession of Tulong, South Tibet—Evidence for early recovery of gymnosperms. Palaeogeography, Palaeoclimatology, Palaeoecology 339–341, 12–24 (2012). [Google Scholar]
- Hochuli P. A. & Vigran J. O. Climate variations in the Boreal Triassic-inferred from palynological records from the Barents Sea. Palaeogeography, Palaeoclimatology, Palaeoecology 290, 20–42 (2010). [Google Scholar]
- Orłowska-Zwolińska T. Palynostratigraphy of the Buntsandstein in section of Western Poland. Acta Palaeontologica Polonica 29, 161–226 (1984). [Google Scholar]
- Bjerager M., Seidler L., Stemmerik L. & Surlyk F. Ammonoid stratigraphy and sedimentary evolution across the Permian–Triassic boundary in East Greenland. Geological Magazine 143, 635–656 (2006). [Google Scholar]
- Sanson-Barrera A. et al. Late Permian-earliest Triassic high-resolution organic carbon isotope and palynofacies records from Kap Stosch (East Greenland). Global and Planetary Change 133, 149–166 (2015). [Google Scholar]
- Balme B. E. Fossil in situ spores and pollen grains: an annotated catalogue. Review of Palaeobotany and Palynology 87, 81–323 (1995). [Google Scholar]
- Hermann E. et al. Climatic oscillations at the onset of the Mesozoic inferred from palynological records from the North Indian Margin. Journal of the Geological Society, London 169, 227–237 (2012). [Google Scholar]
- Visscher H. et al. The terminal Paleozoic fungal event: Evidence of terrestrial ecosystem destabilization and collapse. Proceedings of the National Academy of Sciences of the United States of America 93, 2155–2158 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utting J., Spina A., Jansonius J., McGregor D. C. & Marshall C. E. A. Reworked miospores in the upper Palaezoic and Lower Triassic of the northern circum-polar area and selected localities. Palynology 28, 75–119 (2004). [Google Scholar]
- Peng Y. et al. High-resolution terrestrial Permian–Triassic eventostratigraphic boundary in western Guizhou and eastern Yunnan, southwestern China. Palaeogeography, Palaeoclimatology, Palaeoecology 215, 285–295 (2005). [Google Scholar]
- Eshet Y., Rampino M. R. & Visscher H. Fungal event and palynological record of ecological crisis and recovery across the Permian-Triassic boundary. Geology 23, 967–970 (1995). [Google Scholar]
- Visscher H., Sephton M. A. & Looy C. V. Fungal virulence at the time of the end-Permian biosphere crisis? Geology 39, 883–886 (2011). [Google Scholar]
- Foster C. B., Stephenson M. H., Marshall C., Logan G. A. & Greenwood P. F. A revision of Reduviasporonites Wilson 1962: description, illustration, comparison and biological affinities. Palynology 36, 35–58 (2002). [Google Scholar]
- Spina A., Cirilli S., Utting J. & Jansonius J. Palynology of the Permian and Triassic of the Tesero and Bulla sections (Western Dolomites, Italy) and consideration about the enigmatic species Reduviasporonites chalastus. Review of Palaeobotany and Palynology 218, 3–14 (2015). [Google Scholar]
- Krassilov V. A. & Karasev E. Paleofloristic evidence of climate change near and beyond the Permian–Triassic boundary. Palaeogeography, Palaeoclimatology, Palaeoecology 284, 326–336 (2009). [Google Scholar]
- Tewari et al. The Permian-Triassic palynological transition in the Guryul Ravine section, Kashmir, India: implications for Tethyan-Gondwana correlations. Earth Science Reviews 149, 53–66 (2015).
- Peng Y. & Shi G. R. Life crises on land across the Permian-Triassic boundary in South China. Global and Planetary Change 65, 155–165 (2009). [Google Scholar]
- Ouyang S. & Utting J. Palynology of Upper Permian and Lower Triassic rocks, Meishan, Changxing County, Zhejiang Province, China. Review of Palaeobotany and Palynology 66, 65–103 (1990). [Google Scholar]
- Ouyang S. & Norris G. Earliest Triassic (Induan) spores and pollen from the Junggar Basin, Xinjiang, northwestern China. Review of Palaeobotany and Palynology 106, 1–56 (1999). [Google Scholar]
- Vigran J. O., Mangerud G., Mørk A., Worsley D. & Hochuli P. A. Palynology and geology of the Triassic succession of Svalbard and the Barents Sea. Geological Survey of Norway, Spec. Pub. 14, 1–270 (2014). [Google Scholar]
- Gobet E., Tinner W., Bigler C., Hochuli P. A. & Ammann B. Early Holocene afforestation processes in the lower subalpine belt of the Central Swiss Alps as inferred from macrofossil and pollen records. Holocene, 15, 5, 672–686 (2005). [Google Scholar]
- Lindström S., van de Schootbrugge B., Pedersen G. K., Fiebig J., Nielsen L. H. & Richoz S. No causal link between terrestrial ecosystem change and methane release during the end-Triassic mass extinction. Geology 40, 531–534 (2012). [Google Scholar]
- Vajda V. & Bercovici A. The global vegetation pattern across the Cretaceous–Paleogene extinction interval: a template for other extinction event. Global and Planetary Change 122, 29–49 (2015). [Google Scholar]
- Ovtcharova M. et al. New Early to Middle Triassic U–Pb ages from South China: calibration with ammonoid biochronozones and implications for the timing of the Triassic biotic recovery. Earth and Planetary Science Letters 243, 463–475 (2006). [Google Scholar]