Abstract
Background
Bronchial dysplasia (BD), a presumed precursor of pulmonary squamous cell carcinoma (SCC), rarely progresses to invasive cancer. A high risk cohort at the University of Colorado provided an opportunity to directly sample airway epithelium at mapped sites on successive bronchoscopies. We have hypothesized that persistent dysplastic lesions showing a similar or higher level of dysplasia on follow-up biopsy, are associated with increased risk for the development of SCC.
Methods and Material
Endoscopic biopsies from 188 high risk subjects were histologically classified according to the current WHO classification for BD utilizing a numeric histology score ranging from 1-8 representing normal bronchial mucosa through invasive lung cancer. Differences in follow-up histology scores were compared between sites classified by clinical, histologic and immunohistochemical variables.
Results
Subjects with a higher frequency of sites that persist or progress to high grade dysplasia (≥37.5% persist/progress, N=35 versus <37.5% persist/progress, N=114) show a significant association with development of incident invasive SCC (adjusted hazard ratio: 7.84; 95% confidence interval: 1.56, 39.39), and those with incident lung SCC have adjusted mean follow-up histology scores 1.55 units higher than in subjects without lung cancer. Current smoking, elevated Ki-67 growth fraction, histologic features of angiogenic squamous dysplasia (ASD) and higher histology score in baseline biopsies are significantly associated with increased follow up histology scores.
Conclusions
These results show that persistent BD is associated with the development of invasive SCC. Furthermore, increased expression of Ki-67, the presence of angiogenic change and degree of baseline atypia are associated with persistence of BD.
Keywords: Bronchial dysplasia, Cancer risk, Biomarkers, Chemoprevention, Squamous cell carcinoma of the lung
INTRODUCTION
Bronchial dysplasia (BD) (1) is a presumed precursor lesion of squamous cell carcinoma (SCC) of the lung, and elimination of these lesions has been proposed as a way to prevent invasive SCC of the lung (2). A scoring system for BD that stratifies these lesions by severity of squamous atypia has provided a framework for using histology scores to assess the effect of chemoprevention agents on bronchial mucosa (3-6). Understanding the behavior of BD could provide means to assess risk for the development of lung cancer and elucidate mechanisms underlying progression.
Molecular alterations that parallel those seen in invasive cancer for site specific loss of heterozygosity (LOH), and mRNA, miRNA and immunohistochemical marker expression levels that become more prominent in higher grades of BD has provided data to support a relationship between BD and invasive squamous cell carcinoma (SCC) of the lung (7-10). Additionally, chromosomal aneusomy, gene copy number gains, increased PI3K pathway activation and alterations in telomere length have been shown to be more common in BD from patients with known lung cancer as compared to those without (11-14). A relationship between increased expression of multiple tumor related markers or specific LOH in BD and subsequent development of carcinoma-in-situ (CIS) or SCC has also been shown (15,16). In a few selected cases, an increase in SOX2 amplification has been described as sites of BD progressed in atypia and ultimately developed invasive SCC (17). Progression of atypia or development of cancer has also been related to baseline histologic atypia and other clinical features, but has frequently produced contradictory findings. While some studies have correlated baseline histology with smoking status, more frequent progression of atypia or development of cancer (18-23), others have failed to detect significant relationships with these parameters (15,18,24). Important issues compromising interpretation of these data include use of CIS as a malignant outcome, small numbers of cases per individual report, frequent pre-selection of cases with some cohorts being composed entirely of patients with previous lung or head and neck cancer, confounding of outcome by use of therapeutic intervention, and variable, often short periods of time to follow up assessment of lesions. The limitations associated with these factors were also noted in a recent meta-analysis, in which findings suggested that higher degrees of atypia in BD are associated with more frequent progression or persistence than are lesions of lower grade (25). These findings were not related to development of lung cancer.
Prediction of outcome in BD is of paramount importance to the establishment of reliable, informative screening programs and effective prevention measures. Surveillance techniques such as autofluorescence bronchoscopy have improved sensitivity for detection of BD (26-28) thus providing an opportunity to accurately follow these lesions over time. We have examined the relationship between differences in follow-up histology scores and a variety of parameters and found that more atypical outcomes are associated with subsequent incidence of SCC, increased baseline histologic atypia, smoking status, features of angiogenic squamous dysplasia (ASD) and Ki67 expression levels. We have identified a pattern of persistent BD that defines a subset of subjects with aggressive airway disease and increased risk for development of invasive SCC that may benefit from close follow-up and potential preventive measures.
MATERIALS AND METHODS
Patients at high risk for the development of lung cancer were recruited to bronchoscopy protocols established through the Colorado SPORE in Lung Cancer program. High risk subjects included those with tobacco smoking histories of greater than 20 pack years and those with a personal history of lung cancer or prior cancer of the upper aerodigestive tract. In subjects without a history of cancer, sputum screening was performed and bronchoscopy was offered to individuals with moderate or worse cytologic atypia. Informed consent for enrollment in the protocols and collection of associated clinical data were approved by the Colorado Multiple Institutional Review Board (CoMIRB). Histologic, clinical and other data from subjects for which there was any bronchial site that was sampled on at least two occasions were assembled for this study. Specimens that were collected less than three months after the original baseline biopsy at a specific site were excluded. During the timeframe of specimen collection (1993-2010), two Colorado Lung SPORE sponsored chemoprevention trials were conducted in which all-trans retinoic acid or the prostacyclin analog, Iloprost, were employed as potential chemopreventive agents (3,4). Iloprost, but not all-trans retinoic acid, was shown to be associated with a significant reduction in histology score on follow up biopsy among former smokers as compared with former smokers on the placebo arm of the study. Therefore, all biopsies from treatment arm subjects collected after the Iloprost trial enrollment bronchoscopy were excluded.
Biopsy site classification
Biopsies were assigned a numeric histology score (see figure 1) ranging from 1 to 8 for normal (score =1), basal cell hyperplasia (score = 2), squamous metaplasia without atypia (score = 3), mild dysplasia (score = 4), moderate dysplasia (score = 5), severe dysplasia (score = 6), carcinoma-in-situ (score = 7) and invasive carcinoma (score = 8) with each group being defined by the histologic features described in the WHO classification (1). Baseline histologic score was defined as the diagnosis for the first biopsy at a given site. Follow-up histologic scores were classified into three groups: those with biopsies collected between 3 months to two years, 2-4 years and >4 years after baseline biopsies. If more than one biopsy had been collected during the timeframe of one of these groups, the biopsy with the highest diagnosis was used. Most analyses employed grouping of histology scores into non-dysplastic (scores 1-2), low grade dysplasia (LGD, scores 3-4) and high grade dysplasia (HGD, scores 5-7) histologic groups. In analyses comparing groups defined by a pre-assigned persistent/progressive or regressive classification, persistent/progressive dysplasia (referred to as “persistent” BD throughout manuscript) was defined as any baseline LGD that showed LGD or higher histologic score on follow-up and any baseline HGD that showed HGD or higher histologic score on follow-up unless otherwise noted. Biopsies of histology score 3 or greater were also characterized as angiogenic squamous dysplasias if they showed projections of vascular structures into overlying epithelium as described in previous publications (29) and shown in figures 1E and 1F.
Figure 1.
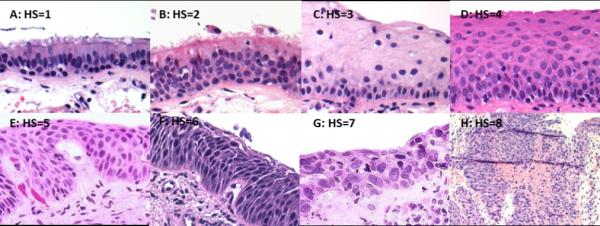
Photomicrographs demonstrating histologic features and histology scores (HS) of bronchial epithelium from sites of non-dysplasia (A = Normal; B = Reserve cell hyperplasia), low grade dysplasia (C = Squamous metaplasia without atypia; D = Mild dysplasia), high grade dysplasia (E = Moderate dysplasia; F = Severe dysplasia; G = Carcinoma-in-situ) and invasive SCC (H). Note vascular papillary projections of angiogenic squamous dysplasia (ASD) in E and F. Magnification: A-G, 400X; H, 100X.
Cancer status was based on subject-level tissue diagnoses with incident carcinoma defined as those cases in which a diagnosis of invasive carcinoma was made 6 months or longer after the date of the baseline bronchoscopy. All other subjects with known lung cancer diagnoses were considered prevalent (or prior) cancer cases. Twenty three of the 41 cases with associated lung carcinomas demonstrated incident carcinomas (in one subject, two synchronous, incident carcinomas occurred in contralateral lung lobes). Fourteen of these were SCC, 4 adenocarcinoma and 6 not otherwise specified (NOS). Tumor diagnoses and sites were established by bronchoscopic biopsy in 8 cases (all SCC), cytologic sampling in 3 (2 SCC, 1 adenocarcinoma) or resection specimens in 7 (4 SCCs, 2 adenocarcinomas and 1 NOS), whereas in 6 cases the method of diagnosis/site were unknown (1 adenocarcinoma and 5 NOS). Among the incident cases, 6 represented second or higher primaries (4 SCC, 1 adenocarcinoma, 1 NOS). Among the 18 cases associated only with prevalent lung carcinomas, 12 were SCC, 5 adenocarcinomas and 1 NOS. Throughout the manuscript, analyses of cancer associated cases indicate whether this group includes prevalent and incident cancers or incident or prevalent cancers alone. Each baseline biopsy was associated with clinical data that were indexed at the time of the baseline biopsy including subject age, gender, smoking status (current, former, never) and pack year smoking history. A subject was considered a former smoker if they had quit at least twelve months before the baseline biopsy was collected.
Immunohistochemistry
All immunohistochemical (IHC) stains had been performed previously in studies assessing the relationship between bronchial histology and marker expression levels (3,4,9). None of the markers, except for a subset of Ki67, had been previously correlated with follow-up histologic scores of biopsy sites. The Ki67 scores represent an expanded set of data, and this analysis of the relationship between expression of this parameter and lesion outcome has not been previously published. IHC analyses utilized marker levels from the first biopsy at a site stained with the marker. Follow-up histology scores were based on biopsies collected subsequent to the biopsy on which the IHC was performed. IHC scoring has been previously described (3,4,9). Briefly, EGFR and HER2 immunostains were classified as normal if the staining was confined to the basal layer of the bronchial epithelium and overexpressed if the staining was seen to extend into the upper half of the epithelium. Ki67, MCM2 and p53 were scored as percent of positive epithelial cells with a goal of counting 400 cells per biopsy from the area that established the diagnosis for that site.
Statistical analysis
Descriptive statistics were used to summarize demographic and clinical characteristics of the data. Mean, standard deviation, median and range were used for continuous variables and frequency and percentage were used for categorical variables. The follow-up time was grouped into three periods: 3 months-2 years, 2-4 years, and greater than 4 years. The dysplasia status was categorized into persistent/progressive or regressive as described in the earlier section. The chi-square test was used to assess the association between grouped baseline histologic score distribution (non-dysplastic, LGD, and HGD) and demographic or clinical characteristics. Multivariable linear mixed effects models were used to evaluate the association between the follow-up histology score, primarily at the window of 3 months-2 years, and predictor variables including dysplasia status, cancer status, tobacco use, smoking status, and biomarker expression, while adjusting for baseline histology score and gender , etc. Correlation among biopsies at different sites from the same patient was accounted for by including a random patient effect. Kaplan-Meier survival curves were obtained for developing SCC based on categorized persistent dysplasia. Cox proportional hazards regression models were used to estimate hazard ratios for developing cancer for persistent dysplasia while adjusting for worst baseline diagnosis score and smoking status only due to a limited number of available events. Persistent dysplasia was modeled as a continuous variable and also as a categorical variable based on quartile cutoff values of its distribution initially and then combining categories if similar survival functions were observed based on the log(-log(survival curves)). The more appropriate functional form between the continuous and categorical was then selected based on the smaller AIC (Akaike information criterion). A bootstrap sampling approach was used to obtain robust hazard ratio estimates and their associated 95% confidence limits. Overall predictive accuracy of the Cox model was assessed using ROC curves following the approach of Heagerty and Zheng (Heagerty and Zheng 2005 survival model predictive accuracy and ROC curves). SAS 9.2 (SAS Inc. Cary, NC, USA) and R (3.12) were used for analysis. P-value <=0.05 was considered statistically significant throughout the paper.
RESULTS
Study subject and biopsy site characteristics
3042 biopsies representing 1170 biopsy sites from 188 subjects were included in the analysis (see supplemental table S1). 402 sites were sampled on three or more occasions. Subjects were more frequently male than female (72.3% male), and ages ranged from 39 to 83 (mean and median 61) years. There were fewer current (79) than former (104) smokers and five subjects were never smokers. 148 biopsy sites were from 41 subjects with diagnoses of lung cancer, and 75 of these sites were from 23 subjects with incident carcinomas. 202 biopsies with histologic features of angiogenic squamous dysplasia (ASD) were associated with follow-up biopsies. Table 1 describes the relationship between baseline histology score and a variety of clinical characteristics. Strong, significant correlation between higher baseline histology score and carcinoma status, cancer subtype, tobacco use, tobacco pack years, gender and age were noted. As described below, several of these variables were also associated with differences in follow-up histology scores although age and gender were never or only infrequently associated with differences in follow-up histology scores, respectively. This lead to the inclusion of all of these parameters except age in multivariable analyses of differences in follow-up histology score presented below. Male gender was adjusted for in those analyses when sample size or number of events was adequate to allow for this.
TABLE 1.
HISTOLOGIC AND CLINICAL CHARACTERISTICS OF BASELINE BIOPSIES
| Non-Dys (%) | LGD (%) | HGD (%) | p-val | |
|---|---|---|---|---|
| LUNG CANCER STATUSa | ||||
| Cancer negative | 598 (59.0) | 179 (17.7) | 237 (23.4) | <0.001 |
| Prevalent lung cancer | 34 (46.6) | 8 (11.0) | 31 (42.5) | |
| Incident lung cancer | 33 (42.9) | 14 (18.2) | 30 (40.0) | |
| LUNG CANCER SUBTYPEb | ||||
| Cancer negative | 598 (58.7) | 179 (17.8) | 237 (23.5) | <0.001 |
| SCC positive | 34 (37.6) | 19 (16.8) | 53 (45.5) | |
| Adenocarcinoma positive | 16 (85.0) | 1 (5.0) | 2 (10.0) | |
| TOBACCO STATUS | ||||
| Current smoker | 231 (42.8) | 138 (25.6) | 171 (31.7) | <0.001 |
| Former smoker | 423 (68.9) | 66 (10.8) | 125 (20.4) | |
| Never smoker | 13 (81.3) | 0 (0.0) | 3 (18.8) | |
| TOBACCO - PACK YEARS | ||||
| Never smoker | 13 (81.3) | 0 (0.0) | 3 (18.8) | <0.001 |
| 0-40 pack years | 273 (64.8) | 73 (17.3) | 75 (17.8) | |
| 40-72 pack years | 238 (48.7) | 107 (21.9) | 144 (29.4) | |
| >72 pack years | 143 (58.6) | 24 (9.8) | 77 (31.6) | |
| GENDER | ||||
| Male | 478 (54.9) | 162 (18.6) | 231 (26.5) | 0.0369 |
| Female | 189 (63.2) | 42 (14.1) | 68 (22.7) | |
| AGE | ||||
| <55 y.o. | 189 (59.1) | 66 (20.6) | 65 (20.3) | 0.0079 |
| 55-65 y.o. | 259 (52.6) | 88 (17.9) | 145 (29.5) | |
| >65 y.o. | 219 (61.2) | 50 (14.0) | 89 (24.8) | |
Correlation between baseline histology and clinical variables.
Cancer status unknown for 6 sites;
Cancer subtype unknown for 31 sites.
Baseline histology is directly correlated with persistence on follow-up biopsy
Each site was evaluated for follow-up histology score at three, two-year interval follow-up time periods. A direct comparison of the frequency of follow-up histology scores at each unique baseline histologic diagnosis indicates two distinct groups of lesions at three months to two years of follow-up: those that show dysplasia (histology score ≥ 3) and those that are non-dysplastic (histology score < 3) on repeat biopsy (figure 2A). These follow-up data show that the proportion of follow-up biopsies with dysplastic morphology increases with increasing baseline histology score. To investigate this relationship, sites with LGD or HGD were compared to those with non-dysplastic histology at baseline and found to show significantly higher frequencies of persistence (follow-up histology score of 3 or greater) in the three months to two years follow-up period with crude risk ratios (RR) of 2.68 (95% CI = 1.66 – 4.34) and 5.22 (95% CI = 3.39 – 8.04) respectively (figure 2B). Persistence was more frequent in the HGD versus the LGD baseline groups in this time interval (crude RR 1.95, 95% CI = 1.21 – 3.14). Significantly higher frequencies of persistence for baseline LGD and HGD compared to non-dysplastic sites were also seen at 2-4 years of follow-up (LGD: crude RR 2.19, 95% CI = 1.15 – 4.18; HGD: crude RR 2.13, 95% CI = 1.14 – 3.95). Although a similar trend was seen in the analysis of sites with follow-up biopsies collected greater than four years post-baseline biopsy (figure 2B), these differences in frequency were not statistically significant possibly due to much smaller number of sites followed for this length of time (see supplemental table S1). Multivariable linear mixed effects model analyses showed that adjusted follow-up histology scores in the three months to two year post-baseline group were on average 0.68 (95% CI, ) units higher in HGD compared to LGD baseline sites and 0.78 (95% CI, ) units higher in LGD compared to non-dysplastic baseline sites following adjustment for tobacco status (current/former/never), pack year tobacco exposure and cancer status (supplemental table S2). Similar, statistically significant, but progressively smaller differences were seen in the 2-4 year and > 4 year follow-up groups with the exception that the mean follow-up histology scores were nearly identical in the baseline LGD and HGD groups for the 2-4 year follow-up period (supplemental table S2).
Figure 2.
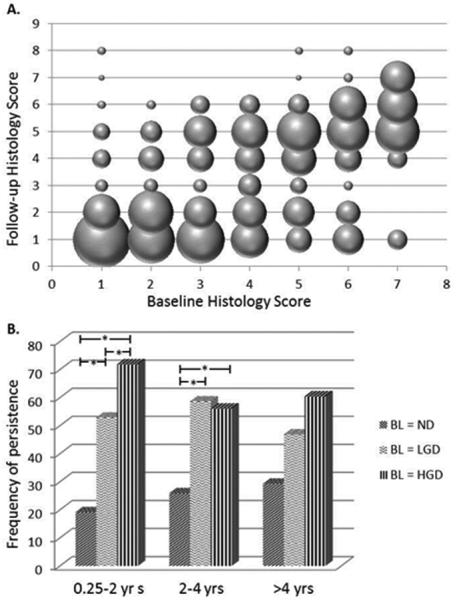
A - Plot of the frequency of specific follow-up histology scores (3 months – 2 years post-baseline biopsy) associated with a given baseline histology score. The area of each sphere is proportional to the percentage of biopsies with the baseline histology score shown on the x-axis that correspond to the follow-up histology score represented on the y-axis. B - The frequency of persistence (follow-up histology score greater than or equal to 3) in non-dysplastic (ND), low grade (LGD) and high grade (HGD) dysplasia baseline (BL) groups at three follow-up time intervals. *p-value < 0.05 after adjustment for age, tobacco status (current/former/never), pack year exposure and cancer status .
Relationship between development of lung cancer and persistence of bronchial dysplasia
Per subject classifications of airway disease were undertaken to determine if persistence of BD could act as an indicator of risk for lung cancer in patients undergoing bronchoscopic evaluation. Two subgroups of cases with baseline BD (LGD or higher) were assessed. These subgroups were composed of cases with one or more BDs at baseline or cases with multiple (2 or more) BDs at baseline. For each of these subgroups two subject level definitions of persistent BD were employed. In one set, persistent BD was defined as presence of LGD or HGD in follow-up biopsies of sites with BD at baseline, and in the second, presence of HGD only on follow-up biopsy of dysplastic baseline sites was considered to represent persistent BD. Chi-square analyses revealed a statistically significant correlation with SCC for the persistent group defined by multiple dysplastic sites persisting as or progressing to HGD but not for the three other alternatively defined persistent BD groups (figure 3A). The same relationship was found when considering persistence or progression to dysplasia for sites of any baseline histology (supplemental figure 1). Cox proportional hazards regression analysis was used to assess the association between the time to diagnosis of incident SCC and percent of sites with HGD on follow-up biopsy. The model fit criteria indicated that the data fit either a continuous or dichotomized model equally well. When considered as a continuous variable with correction for tobacco status and baseline diagnosis, percent of persistent sites showed a hazard ratio of 1.34 (bootstrap 95% CI 1.03, 1.97; p=0.017; (incidence of SCC, n=9)) for every 10% increase in percent of persistent sites. Dichotomization was based on a cutoff (≥37.5% of dysplastic sites showing persistence vs less) selected using AIC as described in the statistical methods section that corresponded to the 75th percentile of the percent of persistent dysplastic sites. A hazard ratio of 7.84 (95% CI: 1.56, 39.39; p=0.003) was obtained for the group in which 37.5% or more sites showed persistence as or progression to HGD compared to the group with less frequent persistence (figure 3B). However, this relationship was not significant and showed a much wider confidence interval using a bootstrap sampling approach (HR=6.63; CI 0.44, >107) indicating that larger numbers of case events are needed to define a reliable cutoff for risk and a more precise estimate of the hazard ratio. Mean baseline histologic score, but not highest baseline histology score, also showed a correlation with SCC in univariable analyses (supplemental figure 2). When mean baseline histology score was treated as a continuous variable, a 2.4 fold (95% CI, 1.2, 4.6) increase in the hazard for developing SCC with each unit increase in histology score was seen. However, a statistically significant cutoff incorporating time to diagnosis for risk of invasive SCC based on mean baseline histology score could not be identified.
Figure 3.
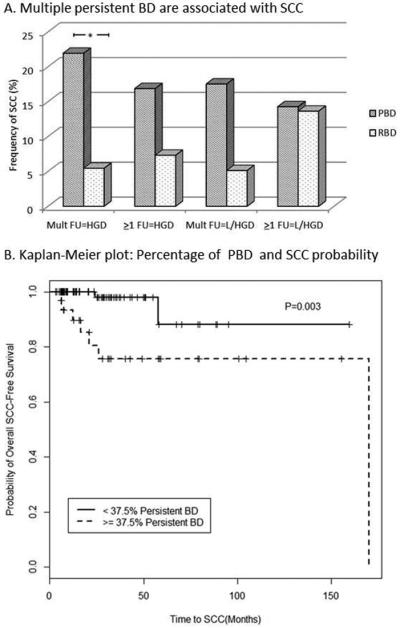
(A) Univariable analysis comparing frequency of SCC using four different definitions of persistent bronchial dysplasia (PBD; RBD = regressive BD) for subjects with dysplasia at baseline (BL HS > 3). The presence of two or more dysplastic sites that persist or progress to HGD (Mult FU=HGD, n=96) shows a statistically significant correlation with presence or development of SCC (p=0.02). When PBD is defined as one or more dysplastic sites with HGD on follow-up (>1 FU=HGD, n= 120; p=0.14), two or more with low or high grade dysplasia on FU (Mult FU=L/HGD, n=96; p=0.07) or one or more with L/HGD on FU (>1 FU=HGD, n=120; p=0.63), PBD is not correlated with SCC. (B) Kaplan-Meier plot demonstrating significantly higher probability of developing SCC in patients (n=35) with 37.5% or more of all sites showing persistence as or progression to HGD compared to those for patients (n=114) with less than 37.5% of their dysplastic sites showing persistence as or progression to HGD (p=0.003 by logrank test).
Comparison of follow-up histology scores in SCC, adenocarcinoma and non-cancer associated cases
A determination of the degree of difference in follow-up histology score for sites from SCC associated cases as compared to those from adenocarcinoma associated cases or cases in which there had been no documented lung cancer was undertaken to characterize the features of persistence that are associated with increased risk for progression to invasive SCC. When considering follow-up histology scores from all prevalent and incident cancer cases together, multivariable linear mixed effects model analyses showed a significant mean increase of 0.82 units (95% CI: 0.32, 1.32) in follow-up histology scores from SCC associated sites compared to the non-cancer associated sites (table 2A). Comparisons of sites from adenocarcinoma associated cases with non-cancer or SCC associated sites did not show significant differences in follow-up histology scores. Compared to non-cancer associated cases, sites from cases with prior SCC but not adenocarcinoma associated cases again showed significantly increased follow-up histology score with a mean difference of 0.73 (95% CI = 0.17 – 1.29) (table 2B). The highest increase in adjusted mean follow-up histology score was seen in the group of biopsies from cases with incident SCC. Incident SCC associated sites showed adjusted mean follow-up histology scores that were 1.55 (CI = 0.80 – 2.30) higher than those from non-cancer sites and the differences were statistically significant regardless of whether sites from cases in which the incident SCC represented a second primary were included or excluded (table 2C). Additionally, incident and prevalent SCC associated sites showed significantly higher frequencies of progression to or persistence as HGD in follow-up biopsies as compared to non-cancer associated sites with the highest frequency seen in sites from patients with incident SCC (supplemental figure 3).
TABLE 2.
CANCER STATUS AND FOLLOW-UP HISTOLOGY SCORE
| A. FOLLOW-UP HISTOLOGY SCORE IN SUBJECTS WITH INCIDENT OR PREVALENT LUNG SCC OR ADENOCARCINOMA VS SUBJECTS WITHOUT ASSOCIATED LUNG CANCER | ||||
|---|---|---|---|---|
| Comparison | Na | Difference in Follow-up HSb | Confidence Interval | p-value |
| SCC vs. No CA | 90, 1022 | 0.82 | 0.32, 1.32 | 0.001 |
| Adenocarcinoma vs. No CA | 19, 1022 | 0.10 | −0.96, 1.16 | 0.855 |
| SCC vs. Adenocarcinoma | 90, 19 | 0.72 | −0.44, 1.88 | 0.221 |
| B. FOLLOW-UP HISTOLOGY SCORES FROM SUBJECTS WITH PREVALENT LUNG SCC OR ADENOCARCINOMA VS SUBJECTS WITHOUT ASSOCIATED LUNG CANCER | ||||
|---|---|---|---|---|
| Prevalent Lung Cancer vs. No Cancer | Na | Difference in Follow-up HSb |
Confidence Interval |
p-value |
| Prevalent SCC vs. No CA | 65, 1022 | 0.73 | 0.17, 1.29 | 0.011 |
| Prevalent Adenocarcinoma vs. No CA | 16, 1022 | 0.01 | −1.04, 1.05 | 0.999 |
| C. FOLLOW-UP HISTOLOGY SCORES FROM SUBJECTS WITH INCIDENT LUNG SCC VS SUBJECTS WITHOUT ASSOCIATED LUNG CANCER | ||||
|---|---|---|---|---|
| SCC vs. No Lung Cancer | Na | Difference in Follow-up HSb | Confidence Interval | p-value |
| Incident SCC, All vs. No CA | 61, 1022 | 1.55 | 0.80, 2.30 | <0.001 |
| Incident SCC, No prior CA vs. No CA | 30, 1022 | 0.99 | 0.08, 1.90 | 0.034 |
Differences in follow-up histology scores (HS) as compared to cases without known lung cancer (No CA) for all prevalent or incident lung cancer cases (A), prevalent lung cancer cases alone (B) or incident SCC cases alone (C).
Number of biopsies (N) for each of the comparator groups respectively.
Data for all analyses are adjusted for baseline diagnosis and tobacco parameters in the multivariable linear mixed effects model.
Relationship between tobacco history and follow-up histology
Tobacco history showed a significant relationship with follow-up histology scores. In multivariable analyses, bronchial sites from current smokers showed a mean 0.37 (95% CI = 0.08 – 0.69) unit increase in follow-up histology score when compared to former smokers (table 3A). In addition, an interaction was noted between smoking status and lung cancer status. The effect of tobacco use was also evaluated by comparisons of subjects divided into three groups characterized by increasing pack year smoking histories (table 3B). Follow-up histology scores at biopsy sites from subjects with 40-72 pack year smoking histories were significantly higher than those from subjects with less than 40 pack years. Interestingly, there was no difference in follow-up histology scores when comparing sites from subjects with more than 72 pack years to those with less than 40 or 40-72 pack year histories.
TABLE 3.
RELATIONSHIP OF TOBACCO USE AND HISTOLOGIC FEATURES TO FOLLOW-UP HISTOLOGY SCORE
| A. TOBACCO STATUS AND FOLLOW-UP HISTOLOGY SCORE | ||||
|---|---|---|---|---|
| Current vs Former Smokers | N (Current, Former) |
Adjusted Difference in Follow-up Histology Scorea |
95% CI | p-Value |
| All Biopsies | 954 (461, 493) | 0.37 | 0.08, 0.69 | 0.0276 |
| No Lung Cancer | 873 (430, 443) | 0.46 | 0.15, 0.76 | 0.005 |
| SCC-associated | 81 (31, 50) | 0.41 | −0.56, 1.38 | 0.41 |
| B. TOBACCO EXPOSURE AND FOLLOW-UP HISTOLOGY SCORE | ||||
|---|---|---|---|---|
| Pack Year Comparison | N | Adjusted Difference in Follow-up Histology Scorea |
95% CI | p-Value |
| 40-72 vs. 1-40 pk yrs | 952 (514, 438) | 0.59 | 0.26, 0.92 | 0.0005 |
| >72 vs. 1-40 pk yrs | 658 (220, 438) | 0.27 | −0.16, 0.70 | 0.22 |
| >72 vs. 40-72 pk yrs | 734 (220, 514) | −0.32 | −0.73, 0.08 | 0.11 |
| C. IHC EXPRESSION AND FOLLOW-UP HISTOLOGIC SCORE | ||||
|---|---|---|---|---|
| Biomarker | N | Adjusted Difference in Follow-up Histology Scorec |
95% CI | p-value |
| Ki67d | 468 | 0.11 | 0.03, 0.20 | 0.0104 |
| p53e | 79 | −0.04 | −0.20, 0.09 | 0.5199 |
| MCM2e | 78 | 0.06 | −0.10, 0.20 | 0.4650 |
| EGFRe | 79 | 0.71 | −0.23, 1.64 | 0.1192 |
| HER2e | 79 | 0.11 | −0.85, 1.09 | 0.8207 |
| D. ASD AND FOLLOW-UP HISTOLOGIC SCORE | ||||
|---|---|---|---|---|
| Raw Mean Follow-up Histology Score |
Adjusted Difference in Follow-up Histology Scoref |
95% CI | p-value | |
| ASD-positive sites | 3.93 | 0.42 | 0.09, 0.78 | 0.016 |
| ASD-negative sites | 3.40 | |||
Analysis of the relationship between follow-up histology score and tobacco use (A, B), immunohistochemical (IHC) marker expression (C) and presence of histologic features of angiogenic squamous dysplasia (ASD) (D).
Outcome differences are adjusted for baseline diagnosis, carcinoma status and tobacco pack years
Outcome differences are adjusted for baseline diagnosis, carcinoma status and tobacco pack years tobacco status.
Changes in histology score for Ki67, p53 and MCM2 are expressed as mean change per 10% increase in number of cells positive for the biomarker; EGFR and HER2 are expressed as mean change in overexpressing sites (staining extends into upper half of epithelium) versus non-overexpressors.
Ki67 data is adjusted for baseline diagnosis, age, gender and tobacco status.
p53, MCM2, EGFR and HER2 data are adjusted for baseline diagnosis only.
The calculated difference in histology score is adjusted for baseline diagnosis, tobacco status and pack year history in the multivariable linear mixed effects models.
Relationship between histologic features and follow-up histology
Immunohistochemical expression of several biomarkers was evaluated to determine if altered expression was associated with an effect on follow-up histology. In univariable analyses, HER2, p53 and MCM2 did not show associations with altered follow-up histology score, but EGFR and Ki67 overexpression were associated with increased follow-up histology scores. We had previously demonstrated that both of these parameters showed a direct correlation with baseline histology score, and therefore adjusted models were employed (9). Ki67 expression retained a significant relationship showing a 0.11 (95% CI = 0.03 – 0.20) unit increase in follow-up histology score per 10% increase in baseline Ki67 positivity (table 3C). Analysis of the relationship between ASD and follow-up histology score was restricted to biopsies with a baseline diagnosis of squamous metaplasia without atypia or higher (histology score of 3 – 7) because the morphologic features of ASD are generally not seen in non-dysplastic lesions. Of note, it was found that there was a significantly higher proportion of high grade dysplasia at baseline in the ASD versus dysplasia without ASD groups (71.9% vs. 53.0% respectively, p < 0.001). Nonetheless, after adjustment for a number of clinical parameters including baseline diagnosis, the presence of histologic features of ASD was also associated with a significant increase in follow-up histology score as compared to sites without features of ASD (table 3D).
DISCUSSION
The potential to use lesion specific changes over time as an indication of risk for the development of invasive SCC of the lung was explored in this study. The findings demonstrated that an increased frequency of sites that show persistence as or progression to HGD was associated with a significant 7.84 (CI = 1.56 – 39.39) fold increase in risk for development of invasive SCC, and indicated that subjects with multiple dysplastic sites that persist or progress to HGD represent a subset of patients with aggressive airway disease. The demonstration that subjects with multiple sites of persistent disease show the strongest association with development of SCC emphasizes the importance of performing a thorough evaluation of the airway, and adds support to the role of field carcinogenesis in the development of invasive lung cancer. Increased risk for invasive SCC has previously been associated with multiple sites of abnormal appearing mucosa by autofluorescence bronchoscopy (AFB) (30). Additionally, the data demonstrate that higher histology scores in follow-up biopsies imply important differences in potential for progression. For instance, cases with dysplastic sites that persisted or progressed to HGD showed a significant increase in risk for development of SCC whereas those in which persistence as low or high grade BD did not, despite the fact that the latter definition of persistence allowed for inclusion of several more cases in the overall evaluation. This finding is similar to those of Alaa, et. al., who demonstrated that the development of new severely dysplastic lesions, regardless of the baseline histology, was more common in subjects that developed invasive cancer or CIS (31). Our data showed similar findings, demonstrating a significant relationship between development of SCC and presence of multiple persistent HGDs when sites of any baseline histology score were included (see supplemental figure 2). However, as discussed below, we and others have observed that CIS often regresses (20). Therefore, establishment of a relationship between persistence of BD and risk for invasive SCC is an important extension of these previous findings.
The demonstration of a relationship between HGD in follow-up biopsies and risk for invasive SCC may also have implications for the management of patients at risk for aggressive airway disease. The tumors that develop in association with persistent BD are more centrally located and are less likely to be associated with identifiable radiographic abnormalities in the early course of disease. Thus, screening for BD and identification of patients at high risk for progression to invasive SCC will likely require a different modality from high resolution CT to be effective. Our findings suggest that multiply sampling the airways may be important and that the employment of a bronchoscopic technique that increases the sensitivity for detection of BD, such as AFB, may be advisable (27, 30). Furthermore, in patients with features of aggressive airway disease, close follow-up would likely be indicated and consideration of potential benefit from preventive therapy might be suggested. With respect to invasive cancer, our study showed that subjects with persistent BD developed invasive cancer both at sites that were associated with and remote from those with baseline dysplastic change. Eight subjects developed incident SCC at sites that were previously biopsied and five at sites that had not been previously biopsied (including one patient that developed synchronous, incident SCCs in two different contralateral lung lobes). Six of the previously biopsied sites showed dysplasia in baseline biopsies, of which two had demonstrated persistence prior to development of SCC (SCC was diagnosed in the second biopsy for the other four). Thus, two SCCs developed at non-dysplastic sites and six others developed at sites that had not been previously sampled suggesting they did not appear abnormal on AFB. This may indicate that chemopreventive rather than local therapy will be necessary to significantly reduce the incidence of SCC in this setting. Finally, the findings also support the use of reduced bronchial histology scores as an informative endpoint in trials evaluating efficacy of potential preventive agents. While we have shown that the frequency of persistent BD is associated with subsequent development of SCC, a potential drawback associated with our analysis is the inclusion of some incident SCC cases in which this tumor represents a second lung primary. Information regarding therapies that patients with prior lung cancer may have received was not available. It is possible that such treatments could influence the course of bronchial dysplasia in the group of patients with prior carcinoma. However, our finding that primary incident SCC is also associated with increased follow-up histology scores further supports a relationship between persistence or progression of BD and risk for the development of invasive SCC.
The association of higher grades of dysplasia at baseline with increased histologic scores on follow-up corroborates findings from the prospective study of Bota, et. al. (21) and the meta-analysis of follow-up data from four different chemoprevention trials performed in the British Columbia Lung Health Study that included over 700 subjects (2). Although different classifications of outcome were used in the latter study, their finding of a 4-5 fold higher rate of progression in sites with baseline diagnoses of moderate or severe dysplasia as compared to those with lower diagnoses is consistent with the findings in our analysis. Strengths of our study that may more firmly establish some of these relationships include fewer numbers of sites coming from subjects with prior lung or head and neck cancers (8.8%), inclusion of sites with lengthy follow-up (48.2% with > 2 years), and confinement of our study group to subjects from the non-treatment protocols or the placebo arm of prevention trials with positive findings. Previously carcinoma-in-situ (CIS) has been reported to progress to invasive frequently with the majority progressing to cancer in some reports (19). In our cohort, nine subjects had 23 sites that showed CIS and had follow-up biopsies. Although histologically normal at the baseline biopsy, one of these sites developed CIS and progressed to invasive SCC seven months later. Additionally, two other subjects developed incident SCCs, but not at their sites of CIS. While16 of 22 (72.7%) CIS sites persisted as HGD, including all of those in cases with associated SCC, six sites in two subjects regressed to non-dysplastic histology including five that were followed over a course of 35 months and were re-biopsied 1-3 times. Taking the biopsy with the highest diagnosis prior to development of SCC, one site with CIS at baseline (1/22, 4.54%), five sites with baseline moderate or severe dysplasia (5/282, 1.77%), one with baseline LGD (1/204, 0.49%) and four with non-dysplastic baseline diagnoses progressed to SCC (4/667, 0.60%). Although the overall number of CIS lesions is small in this cohort, the findings support the aggressive nature that other publications have found to be associated with these lesions, but also suggests that the rate of progression is not high and document regression of CIS.
Our data show that tobacco use has an impact on the course of BD with current tobacco users having higher follow-up histology scores than former tobacco users. These findings compliment the findings of Clement, et al. in which duration of smoking history was found to correlate with increased incidence of bronchial dysplasia (32). This information could be useful clinically for physicians counseling their patients to quit tobacco use as a measure to prevent the development of lung cancer.
Angiogenic squamous dysplasias were also shown to be associated with an increased level of atypia on follow-up biopsy. Angiogenesis is well established as a prognostic factor in invasive carcinoma, and we have previously shown that expression of vascular endothelial growth factor increases with higher grades of BD (33). Furthermore, our recent analysis of vandetanib, the VEGFR2 inhibitor with multi-target inhibitory capacity, showed preventive activity of this agent in a mouse model of lung carcinogenesis (34). These findings also correlate with previous work that has demonstrated more frequent ASD in subjects with lung cancer than in those without (35). Angiogenic changes could support an increased level of epithelial cell proliferation that may be important in promoting BD persistence and progression. Given that poor vascular integrity has been associated with VEGF dominant neoangiogenesis (36), it is also possible that ASD lesions are associated with an altered microenvironment that promotes progression. Additionally, our IHC analyses suggest that higher levels of expression of Ki67 could also serve as biomarkers of increased risk in BD.
The results of this study suggest that an important subset of aggressive airway disease is represented by cases that show the presence of multiple dysplastic lesions that persist or progress to HGD, and demonstrate that in patients with this presentation there is increased risk for invasive SCC. Further characterization of these persistent lesions should allow for the development of more precise predictive markers. Furthermore, obtaining an understanding of the biologic characteristics that drive these BD with a high risk for progression to invasive lung cancer will help identify effective targets for prevention.
Supplementary Material
Acknowledgments
GRANT SUPPORT: Lung Specialized Programs of Research Excellence P50 CA058187 and Cancer Center Support Grant P30 CA046934 (all authors received P30 CA046934)
Footnotes
CONFLICT OF INTEREST: Dr. Merrick has no conflicts of interest to report
REFERENCES
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Thymus and Heart. 4th. IARC; Lyon: 2015. WHO Classification of Tumours of the Lung, Pleara; pp. 59–63. p. [DOI] [PubMed] [Google Scholar]
- 2.Ishizumi T, McWilliams A, Macaulay C, Gazdar A, Lam SS. Natural history of bronchial preinvasive lesions. Cancer Metastasis Rev. 2010;29:5–14. doi: 10.1007/s10555-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keith RL, Blatchford PJ, Kittelson J, Minna JD, Kelly K, Massion PP, et al. Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev Res (Phila) 2011;4:793–80. doi: 10.1158/1940-6207.CAPR-11-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly K, Kittelson J, Franklin WA, Kennedy TC, Klein CE, Keith RL, et al. A randomized phase II chemoprevention trial of 13-CIS retinoic acid with or without alpha tocopherol or observation in subjects at high risk for lung cancer. Cancer Prev Res (Phila) 2009;5:440–9. doi: 10.1158/1940-6207.CAPR-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam S, leRiche JC, McWilliams A, MacAulay C, Dyachkova Y, Szabo E, et al. A randomized phase IIb trial of pulmicort turbuhaler (budesonide) in persons with dysplasia of the bronchial epithelium. Clinical Cancer Research. 2004;10:6502–6511. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 6.Lam S, McWilliams A, Leriche J, MacAulay C, Wattenburg L, Szabo E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiology Biomarkers & Prevention. 2006;15:1526–1531. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 7.Wistuba II, Behrens C, Milchgrub S, Bryant D, Hung J, Minna JD, et al. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene. 1999;18:643–650. doi: 10.1038/sj.onc.1202349. [DOI] [PubMed] [Google Scholar]
- 8.Wistuba II, Behrens C, Virmani Ak, Mele G, Milchgrub S, Girard L, et al. High resolution chromosome 3p allelotyping of human lung cancer and bronchial epithelium reveals multiple, discontinuous sites of 3pallele loss and three regions of frequent breakpoints. Cancer Research. 2000;60:1949–1960. [PubMed] [Google Scholar]
- 9.Merrick DT, Kittelson J, Winterhalder R, Kotantoulas G, Ingeberg S, Keith RL, et al. Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: evaluation of potential targets for chemoprevention of lung cancer. Clin Cancer Res. 2006;12:2281–8. doi: 10.1158/1078-0432.CCR-05-2291. [DOI] [PubMed] [Google Scholar]
- 10.Mascaux C, Laes JF, Anthoine G, Haller A, Ninane V, Burny A, et al. Evolution of microRNA expression during human bronchial squamous carcinogenesis. Eur Respir J. 2009;33:352–9. doi: 10.1183/09031936.00084108. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson S, Varella-Garcia M, Miller Y, Wolf HJ, Byers T, Braudrick S, et al. Chromosomal aneusomy in bronchial high grade lesions is associated with invasive lung cancer. American Journal of Respiratory and Critical Care Medicine. 2008;177:342–347. doi: 10.1164/rccm.200708-1142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massion P, Zou Y, Uner H, Kiatsimkul P, Wolf HJ, Baron AE, et al. Recurrent genomic gains in preinvasive lesions as a biomarker of risk for lung cancer. PLoS ONE. 2009;4:e5611. doi: 10.1371/journal.pone.0005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, et al. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2:1–11. doi: 10.1126/scitranslmed.3000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lantuejol S, Raynaud C, Salameire D, Gazzeri S, Moro-Sibilot D, Soria J-C, et al. Telomere maintenance and DNA damage response during lung carcinogenesis. Cllin Cancer Res. 2010;16:2979–2988. doi: 10.1158/1078-0432.CCR-10-0142. [DOI] [PubMed] [Google Scholar]
- 15.Jeanmart, Lantuejoul S, Fievet F, Moro D, Sturm N, Brambilla C, et al. Value of immunohistochemical markers in preinvasive bronchial lesions in risk assessment of lung cancer. Clin Cancer Res. 2003;9:2195–2203. [PubMed] [Google Scholar]
- 16.Salaun M, Sesboue R, Moreno-Swire S, Metayer J, Bota S, Bourguignon J, et al. Molecular predictive factors for progression of high-grade preinvasive bronchial lesions. American Journal of Respiratory and Critical Care Medicine. 2008;177:880–886. doi: 10.1164/rccm.200704-598OC. [DOI] [PubMed] [Google Scholar]
- 17.McCaughan F, Pole JCM, Bankier AT, Konfortov BA, Carroll B, Falzon M, et al. Progressive 3q Amplification Consistently Targets SOX2 in Preinvasive Squamous Lung Cancer. Am J Resp Crit Care Med. 2010;182:83–91. doi: 10.1164/rccm.201001-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breuer RH, Pasic A, Smit EF, van Vliet E, Vonk Noordegraaf A, Risse EJ, et al. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res. 2005;11:537–543. [PubMed] [Google Scholar]
- 19.Venmans B, van Boxem A, Smit E, Postmus P, Sutedja T. Outcome of bronchial carcinoma in-situ. Chest. 2000;117:1572–1576. doi: 10.1378/chest.117.6.1572. [DOI] [PubMed] [Google Scholar]
- 20.Moro-Sibilot D, Fievet F, Jeanmart M, Lantuejoul S, Arbib F, Laverribre MH, et al. Clinical prognostic indicators of high-grade pre-invasive bronchial lesions. Eur Respirology Journal. 2004;24:24–29. doi: 10.1183/09031936.04.00065303. [DOI] [PubMed] [Google Scholar]
- 21.Bota S, Auliac JB, Paris C, Metayer J, Sesboue R, Nouvet G, et al. Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. American Journal of Respiratory and Critical Care Medicine. 2001;164:1688–1693. doi: 10.1164/ajrccm.164.9.2012147. [DOI] [PubMed] [Google Scholar]
- 22.Salaun M, Bota S, Thiberville L. Long-term followup of severe dysplasia and carcinoma in-situ of the bronchus. J Thorac Oncol. 2009;4:1187–1188. doi: 10.1097/JTO.0b013e3181b28f44. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino H, Shibuya K, Chiyo M, Iyoda A, Yoshida S, Sekine Y, et al. Biological features of bronchial squamous dysplasia followed up by autofluorescence bronchoscopy. Lung Cancer. 2004;46:187–196. doi: 10.1016/j.lungcan.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Pasic A, van Vliet E, Breuer RH, Risse EJ, Snijders PJ, Postmus PE, et al. Smoking behavior does not influence the natural course of pre-invasive lesions in bronchial mucosa. Lung Cancer. 2004;45:153–154. doi: 10.1016/j.lungcan.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee AK. Preinvasive lesions of the bronchus. J Thorac Oncol. 2009;4:545–551. doi: 10.1097/JTO.0b013e31819667bd. [DOI] [PubMed] [Google Scholar]
- 26.Lam S, Kennedy T, Unger M, Miller YE, Gelmont D, Rusch V, et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest. 1998;113:696–702. doi: 10.1378/chest.113.3.696. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch FR, Prindiville SA, Miller YE, Franklin WA, Dempsey EC, Murphy JR, et al. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: A randomized study. J Natl Cancer Inst. 2001;93:1385–1391. doi: 10.1093/jnci/93.18.1385. [DOI] [PubMed] [Google Scholar]
- 28.Edell E, Lam S, Pass H, Miller YE, Sutedja T, Kennedy T, et al. Detection and localization of intraepithelial neoplasia and invasive carcinoma using fluorescence-reflectance bronchoscopy: An international, multi-center clinical trial. J Thorac Oncol. 2009;4:49–54. doi: 10.1097/JTO.0b013e3181914506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keith RL, Miller YE, Gemmill RM, Drabkin HA, Dempsey EC, Kennedy TC, et al. Angiogenic squamous dysplasia in bronchi of individuals at high risk for lung cancer. Clin Cancer Res. 2000;6:1616–25. [PubMed] [Google Scholar]
- 30.Pasic A, Vonk-Noordegraaf A, Risse EK, Postmus PE, Sutedja TG. Multiple suspicious lesions detected by autofluorescence bronchoscopy predict malignant development in the bronchial mucosa in high risk patients. Lung Cancer. 2003;41:295–301. doi: 10.1016/s0169-5002(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 31.Alaa MRM, Shibuya K, Fujiwara T, Wada H, Hoshino H, Yoshida S, et al. Risk of lung cancer in patients with preinvasive bronchial lesions followed by autofluorescence bronchoscopy and chest computed tomography. Lung Cancer. 2011;72:303–308. doi: 10.1016/j.lungcan.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Clément-Duchênea C, Alla F, Gauchotte G, Marie B, Carnin C, Menard O, et al. Is there a relationship between the presence of lung mucosa preinvasive lesions and lung cancer incidence? Influence of tobacco consumption. Lung Cancer. 2014;84:134–138. doi: 10.1016/j.lungcan.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Merrick DT, Haney J, Petrunich S, Sugita M, Miller YE, Keith RL, et al. Overexpression of vascular endothelial growth factor and its receptors in bronchial dysplasia demonstrated by quantitative RT-PCR analysis. Lung Cancer. 2005;48:31–45. doi: 10.1016/j.lungcan.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 34.Karoor V, Le M, Merrick D, Dempsey EC, Miller YE. Cancer Vascular endothelial growth factor receptor 2-targeted chemoprevention of murine lung tumors. Prev Res (Phila) 2010;3:1141–7. doi: 10.1158/1940-6207.CAPR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karimi S, Mohammadi F, Khodadad K, Sadr M, Seyfollahi L, Masjedi MR. Relationship between angiogenic squamous dysplasia and bronchogenic carcinoma in patients undergoing white light bronchoscopy. Can Respir J. 2012;19:201–208. doi: 10.1155/2012/343954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenberg JI, Cheresh DA. VEGF as an inhibitor of tumor vessel maturation: implications for cancer therapy. Expert Opin Biol Ther. 2009;9:1347–1356. doi: 10.1517/14712590903208883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



