Preface
The survival and well-being of all species requires appropriate physiological responses to environmental and homeostatic challenges. The reestablishment and maintenance of homeostasis entails the coordinate activation and control of neuroendocrine and autonomic stress systems. These collective stress responses are mediated via largely overlapping circuits in the limbic forebrain, hypothalamus and brainstem, so that the respective contribution of the neuroendocrine and autonomic systems is tuned in accordance with stressor modality or intensity. Limbic regions that are responsible for regulating stress responses intersect with circuits that are responsible for memory and reward, providing a means to tailor the stress response with respect to prior experience and anticipated outcomes.
Introduction
Adaptation in the face of stress is a major priority for all organisms. Stress can be broadly defined as a real or anticipated disruption of homeostasis or an anticipated threat to well-being. Stressor-related information from all major sensory systems (e.g. interoceptive cues, such as blood volume and osmolarity, and/or exteroceptive cues, such as the smell of a predator) is conveyed to the brain, which recruits neural and neuroendocrine systems (effectors) to minimize the net cost to the animal. The physiological response to stress involves an efficient and highly-conserved set of interlocking systems that aims to maintain physiologic integrity even in the most demanding of circumstances.
The autonomic nervous system (ANS) (Text Box 1) provides the most immediate response to stressor exposure via its sympathetic and parasympathetic arms that provoke rapid alterations in physiological states through neural innervation of end organs. For example, the sympatho-adrenomedullary arm can rapidly increase heart rate and blood pressure (in seconds) by excitation of the cardiovascular system1. Importantly, excitation of the ANS wanes quickly — due to reflex parasympathetic activation —, resulting in short-lived responses.
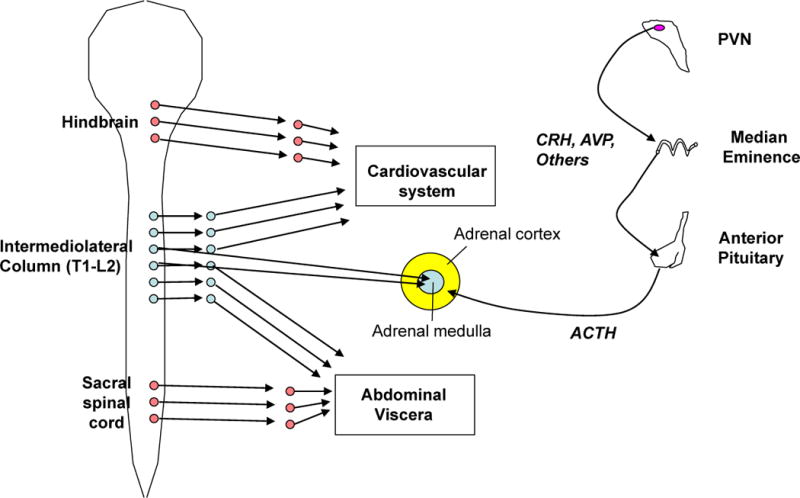
Box 1. HPA axis and autonomic nervous system responses to stress.
The sympatho-adrenal medullary (see figure, left) and hypothalamic-pituitary-adrenocortical (HPA, see figure, right) axes are the primary systems for the maintenance or reinstatement of homeostasis during stress. Stressor exposure results in activation of preganglionic sympathetic neurons in the intermediolateral cell column of the thoracolumber spinal cord (shown in blue). These preganglionic neurons project to pre- or para-vertebral ganglia that in turn project to end organs, and to chromaffin cells of the adrenal medulla. This sympathetic activation represents the classic ‘fight or flight’ response that was first characterized by Walter Cannon and colleagues in the early 20th century152; it generally increases circulating levels of adrenaline (primarily from the adrenal medulla) and noradrenaline (primarily from sympathetic nerves), heart rate and force of contraction, peripheral vasoconstriction, and energy mobilization. Parasympathetic tone can also be modulated during stress. In the parasympathetic system (shown in red), activation of craniosacral preganglionic nuclei activates postganglionic nuclei located in or near the end organs they innervate; parasympathetic actions are generally opposite those of the sympathetic system.
For the HPA axis, stressor exposure activates hypophysiotropic neurons in the PVN that secrete releasing hormones, such as corticotrophin releasing hormone and vasopressin, into the portal circulation of the median eminence. These releasing hormones act on the anterior pituitary to promote the secretion of adrenocorticotropic hormone, which in turn acts on the inner adrenal cortex (i.e. the zona fasciculata) to initiate the synthesis and release of glucocorticoid hormones (e.g. corticosterone in rats and cortisol in humans). Circulating glucocorticoids then promote the mobilization of stored energy and potentiate numerous sympathetically-mediated effects, such as peripheral vasoconstriction. Moreover, the adrenal cortex is directly innervated by the sympathetic nervous system, which can facilitate corticosteroid release153. Thus, the HPA axis and sympathetic system have largely complementary actions throughout the body, including energy mobilization and maintenance of blood pressure during stress.
Activation of the hypothalamo-pituitary-adrenocortical (HPA) axis results in elevations in circulating glucocorticoids (Text Box 1). Peak plasma glucocorticoid levels occur tens of minutes after initiation of stress2. The two-step hormonal mechanism of HPA induction (Box 1) is sluggish relative to the latency of the synaptic mechanisms driving sympatho-adrenomedullary activation, and ensures an amplified and relatively protracted secretory episode.
The brain triggers stress responses that are commensurate with the nature of the stimulus. Physical stressors — blood loss, infection, pain — require an immediate ‘systemic’ reaction that is triggered by reflexive mechanisms. The brain also responds to non-physical or ‘psychogenic’ stressors based on prior experience or innate programs3. These responses require processing in forebrain, and can occur in anticipation of or in reaction to stressful events.
This Review addresses the brain circuits that regulate ANS and HPA axis responses to stress. The first section reviews the neurocircuitry, focusing on ascending inputs from the brainstem, descending influences from the limbic forebrain, and hypothalamic mechanisms that integrate limbic and brainstem input with respect to homeostatic feedback (Figure 1). The second section presents a case for reorganization of stress-control circuitry in the chronically stressed brain, involving recruitment of some regions and diminished impact of others. The final section discusses overlap of stress circuits and those controlling memory and reward, an anatomical arrangement that provides opportunity for mutual interaction in the ultimate neural interpretation of stressor significance.
Figure 1. General scheme of brain acute stress-regulatory pathways.
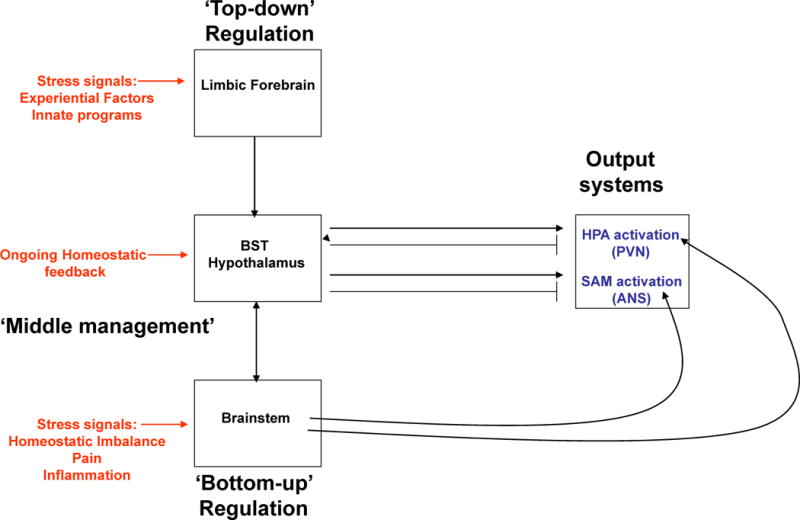
Stressors activate brainstem and/or forebrain limbic structures. The brainstem is able to generate rapid hypothalamus-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) responses via direct projections to hypophysiotrophic neurons in the paraventricular nucleus of the hypothalamus (PVN) or to preganglionic autonomic neurons (bottom-up regulation). In contrast, forebrain limbic regions have no direct connections with the HPA axis or the ANS and thus require intervening synapses prior to accessing autonomic or neuroendocrine neurons (top-down regulation). A high proportion of these intervening neurons are located in hypothalamic nuclei that are also responsive to homeostatic status, providing a mechanism by which the descending limbic information can be modulated according to the physiological status of the animal (middle management).
Triggering stress responses: Brainstem/hypothalamic mechanisms
Brainstem systems
The brainstem receives inputs that signal major homeostatic perturbations such as blood loss, respiratory distress, visceral or somatic pain and inflammation3. Sympathetic responses to these inputs involve reflex arcs that communicate with areas in the medulla (e.g., rostral ventrolateral medulla) and preganglionic sympathetic neurons in the intermediolateral cell column of the spinal cord1 (Figure 2). Coordinate alteration of the parasympathetic branch of the ANS also occurs following stress, altering ‘vagal tone’ to the heart and lungs1 and helping to control the duration of autonomic responses. This parasympathetic response to stress is mediated by the nucleus ambiguus and dorsal motor nucleus of the vagus nerve, possibly via input from the nucleus of the solitary tract (NTS)1 (Figure 2). In addition, medullary and spinal cord systems inform higher-order autonomic integrative sites in the hindbrain (e.g., raphe pallidus, lateral parabrachial nucleus and Kölliker-Fuse nucleus), midbrain and forebrain (e.g., dorsomedial hypothalamus)1, which modulate the autonomic response to stressors in accordance with descending information from hypothalamus and limbic forebrain. Although all of these circuits participate in autonomic integration, their precise role(s) in stress-induced responses is not yet defined.
Figure 2. Brain circuitry regulating autonomic stress responses.
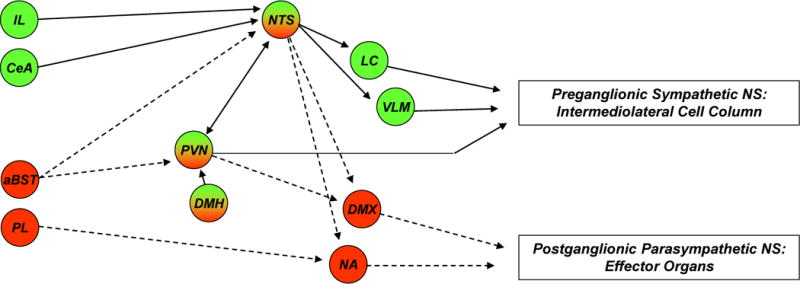
Stress-induced pre-autonomic outflow originates in multiple brain areas. Colors denote brain regions that are implicated in sympathetic activation (blue), parasympathetic activation (red) or both (bicolored). The paraventricular nucleus of the hypothalamus (PVN) has substantial projections to both sympathetic and parasympathetic nuclei, including the nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus nerve (DMX), intermediolateral cell column (IML), locus coeruleus (LC) and ventrolateral medulla (VLM) (latter two not shown for clarity). The rostral VLM, LC, and PVN provide direct innervation of the IML and are thought to initiate sympathetic responses. These NTS in turn receive direct input from neurons in the infralimbic cortex (IL), central amygdala (CeA) and PVN. Other hypothalamic regions, most notably the dorsomedial hypothalamus (DMH), modulate ANS activation via connections with the PVN (and possibly other descending pathways) (see text). Parasympathetic outflow is mediated largely by descending outflow from the DMX and nucleus ambiguous (NA) (colored red) and is under the direct influence of the prelimbic cortex (PL), PVN and possibly other descending relays (see text). Parasympathetic effects of the anterior bed nucleus of the stria terminalis (aBST) are likely mediated by relays in the PVN or the NTS. The anatomical complexity of ANS integration is underscored by the mixing of sympathetic and parasympathetic projection neurons in individual nuclei.
Signals of homeostatic imbalance in the brainstem also lead to activation of the HPA axis: ascending brainstem (and perhaps spinal) pathways project heavily to the parvocellular divisions of the paraventricular nucleus of the hypothalamus (PVN) (Figure 3). For example, catecholaminergic (e.g., noradrenaline and adrenaline) projections to the hypophysiotrophic zone of the PVN originate in the NTS and C1–C3 regions4, 5 and participate in HPA activation (see6). NTS projections to the same area release additional neuroactive factors (including neuropeptide Y, glucagon-like peptide 1, inhibin β, somatostatin and enkephalin7–9) that can regulate HPA activation. In some but not all NTS neurons these factors are co-localized with noradrenaline and adrenaline8.
Figure 3. Brain circuitry regulating HPA axis stress responses.
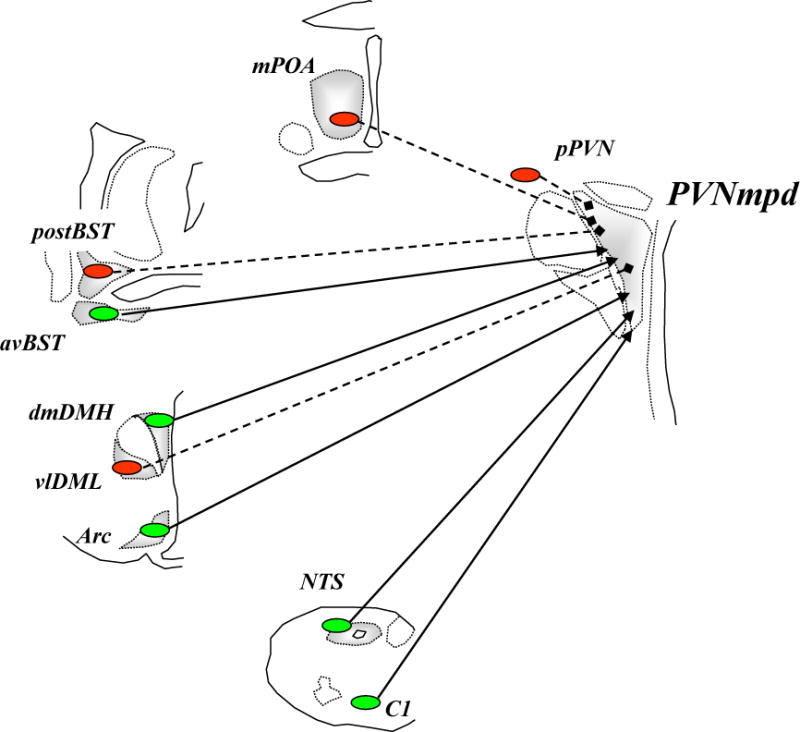
Stress-induced activation of the dorsal part of medial parvocellular paraventricular nucleus of the hypothalamus (PVNmpd) originates in several brain regions (excitatory inputs colored blue with solid lines and inhibitory inputs (GABA) colored red with dashed lines). The paraventricular nucleus of the hypothalamus (PVN) receives direct noradrenergic, adrenergic and peptidergic innervation from the nucleus of the solitary tract (NTS). The dorsomedial component of dorsomedial hypothalamus (dmDMH) and arcuate nucleus (Arc) provide intrahypothalamic stress excitation. The anterior part of the bed nucleus of the stria terminalis (BST), particularly the anteroventral nucleus of the BST (avBST), activates HPA axis stress responses. The PVN also receives stress-excitatory drive from the avBST, dorsal raphe, tuberomammillary nucleus, supramammillary nucleus, and spinal cord, among others (omitted in the interest of space). Activation of the PVNmpd is inhibited by numerous hypothalamic circuits, including the medial preoptic area (mPOA), ventrolateral component of dorsomedial hypothalamus (vlDMH) and local neurons in the peri-PVN region (pPVN), encompassing the PVN surround and the subparaventricular zone. The posterior subregions of the bed nucleus of the stria terminalis (pBST) provides a prominent forebrain inhibition of HPA axis responses; the majority of these inputs are GABAergic.
Destruction of ascending noradrenaline/adrenaline neurons reduces HPA-axis responses to stimuli that signal homeostatic perturbations (e.g., hypoglycemia or interleukin 1β injection)10, 11, but does not affect psychogenic responses, leading to the hypothesis that ascending catecholaminergic pathways mediate systemic-stress responses3. However, some non-catecholaminergic NTS cell groups (e.g., glucagon-like peptide 1 neurons)12 are involved in the generation of HPA responses to both psychogenic and systemic stressors, suggesting some degree of functional specialization among NTS-PVN pathways.
The parvocellular PVN also receives serotonergic innervation from the median raphe nuclei in the midbrain. Serotonin activates the HPA axis13 through activation of serotonin 2A receptors on PVN neurons14. Serotonergic fibers project equally to the PVN and surrounding regions15, raising the possibility that serotonin also influences local circuit neurons projecting into the PVN.
Circumventricular organs: integration of peripheral signals
Components of the lamina terminalis system in the forebrain (i.e. the median preoptic nucleus, the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis) respond to perturbations in fluid and electrolyte balance and blood pressure. This system is essential for the central regulation of blood pressure by angiotensin II. The SFO16 has direct angiotensin II-containing projections to the medial parvocellular PVN, where it stimulates HPA-axis activity via activation of the angiotensin II type-I receptor17. The lamina terminalis also projects to other hypothalamic structures, including the anteroventral preoptic nucleus, DMH and preautonomic PVN18, through which it can initiate stress-induced changes in cardiovascular activity19.
Paraventricular and Dorsomedial Nuclei
Among several hypothalamic nuclei directly involved in the regulation of HPA axis and autonomic responses to stressors, the PVN stands out as a principal integrator of stress signals. The PVN houses distinct populations of neurons that project to the median eminence and to autonomic targets in the brainstem and spinal cord, such as the intermediolateral cell column, parabrachial nucleus, dorsal motor nucleus of the vagus and NTS20. Transneuronal tracing studies indicate that parasympathetic and sympathetic projection neurons are intermingled in the PVN, suggesting input into both arms of ANS function21.
The PVN is heavily innervated by GABAergic inputs22, which provide a substantial inhibitory tone23, 24 (Figures 3 and 4). Some of the GABAergic innervation to the PVN comes from neurons in the peri-PVN region25, which in turn is targeted by several limbic brain regions, enabling it to translate limbic information into modulation of HPA axis or autonomic activation26.
Figure 4. Organization of limbic outputs.
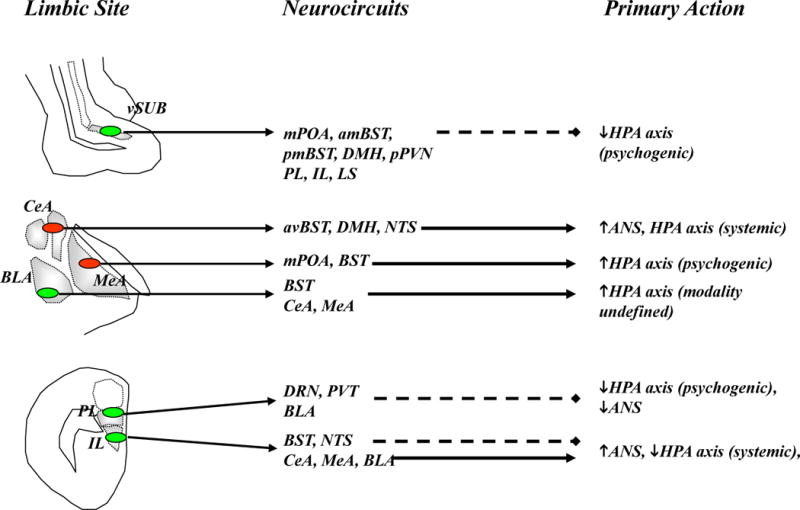
Limbic modulation of stress responses occurs predominantly via oligosynaptic inputs to the in the paraventricular nucleus of the hypothalamus (PVN) and other preautonomic brain regions. Excitatory inputs are colored blue with solid lines and inhibitory inputs (GABA) are colored red with dashed lines. Top: The ventral subiculum (vSUB) coordinates hippocampal stress output by providing glutamatergic input to primarily inhibitory PVN relays, thereby limiting psychogenic stress responses. Middle: GABAergic projections from the central amygdala (CeA) regulate responses to systemic stressors, whereas those from the medial amygdala (MeA) preferentially modulate responses to psychogenic stressors. Through glutamatergic projections within and outside the amygdala, the basolateral amygdala (BLA) plays a role in both the acute response to psychogenic stress and in chronic stress regulation. Bottom: The prelimbic cortex (PL) inhibits responses to psychogenic stress, and this inhibition is mediated predominantly by glutamatergic projections to inhibitory PVN relays. In contrast, the infralimbic cortex (IL) activates autonomic and possibly HPA axis responses to psychogenic stress, perhaps via direct (nucleus of the solitary tract, NTS) or indirect (CeA) projections. Abbreviations: anteriomedial BST (amBST), anteroventral BST (avBST), bed nucleus of the stria terminalis (BST), dorsal raphe nucleus (DRN), dorsomedial hypothalamus (DMH), lateral septum (LS), medial preoptic area (mPOA), thalamic paraventricular nucleus (PVT), peri-PVN (pPVN), posteromedial BST (pmBST), ventral subiculum (vSub).
The DMH regulates autonomic and perhaps also HPA-axis responses to psychogenic stimuli. Local stimulation of the DMH increases heart rate, blood pressure and HPA-axis responses to a psychological stressor27, whereas inhibition attenuates stress-induced increases in heart rate and blood pressure28. However, inactivation of the DMH does not affect cardiovascular responses to hemorrhage28, indicating that hypovolaemic stimuli are processed elsewhere (most likely through brainstem reflex pathways). The DMH may also be involved in gating PVN activation: local inhibition of the dorsal DMH reduces ACTH release and neural excitation (as reflected by immediate early gene (Fos) activation29) in the parvocellular PVN to psychogenic, but not systemic stimuli30. Moreover, the ventral DMH inhibits neuronal activity in the PVN31, indicating that the DMH contains anatomically segregated neuronal populations that activate or inhibit HPA-axis activity (Figure 3).
Top-down regulation: limbic stress circuits
Both psychogenic and systemic stimuli are processed in multiple limbic forebrain structures, including the amygdala, hippocampus and prefrontal cortex. These regions receive associational information from subcortical and cortical areas that are involved in higher-order sensory processing (e,g., olfactory nuclei, pirifirm cortex, insular cortex) and memory (medial septum, entorhinal cortex, cingulate cortices), as well as ascending inputs from sites involved in attention and arousal (e.g., locus coeruleus, raphe nuclei). The output from these limbic structures converges on crucial subcortical relay sites, providing for downstream processing of limbic information3. These limbic regions work in parallel to influence the activation of the HPA-axis, and likely perform similar functions in the autonomic responses to stress.
Amygdala
The amygdala is structurally complex with numerous downstream targets that modulate autonomic and neuroendocrine stress responses (Figure 4). The central nucleus of the amygdala (CeA) has received considerable attention as a key node for stress integration, given its involvement in autonomic regulation and its association with stress-related behaviors (specifically, fear responses)32. The CeA is differentially activated by homeostatic disruption33, 34 and systemic35, but not psychogenic36, 37 stressors. Nonetheless, CeA lesions impair bradycardic responses during exposure to psychological stressors38, 39, suggesting a role in integrating the autonomic components of psychogenic stress. Overall, the role of the CeA appears specific to stimulus modality, and may be preferentially weighted toward autonomic rather than HPA-axis responses to stress.
The medial (MeA) and basolateral (BLA) amygdala nuclei are preferentially activated by psychological stressors33, 40, 41: lesions of the MeA produce selective deficits in HPA axis responses to psychogenic but not homeostatic stressors36. and BLA lesions dampen HPA-axis responses to restraint42. The impact of the MeA and BLA on HPA responses is likely mediated by extensive interactions with intervening PVN-projecting neurons, as there are few direct connections between the PVN and these amygdala nuclei43. The roles of the MeA and BLA in regulation of autonomic stress responses have yet to be definitively tested; however, based on the paucity of MeA and BLA projections to principal autonomic output nuclei, it appears unlikely that these regions directly modulate autonomic stress responses to a significant extent.
Hippocampus
Numerous studies link the hippocampus with inhibition of the HPA axis3, 44. Hippocampal stimulation decreases glucocorticoid secretion in rats and humans45, 46, whereas hippocampal damage increases stress-induced and in some cases, basal glucocorticoid secretion3, 44. Notably, lesion effects are most pronounced during the recovery phase of stress-induced glucocorticoid secretion, implicating the hippocampus in regulating the termination of stress-initiated HPA responses.
Hippocampal regulation of the HPA axis is region- and stressor-specific. The inhibitory effects of the hippocampus on the PVN are subserved by a relatively circumscribed population of neurons in the ventral subiculum47 (Figure 4). Lesions of this area result in increased corticosterone release following psychogenic, but not systemic stressors47, consistent with context-specific modulation of stress responses by the hippocampus.
The hippocampus also influences autonomic tone. Hippocampal stimulation decreases heart rate, blood pressure and respiratory rate in awake rats, effects that are blocked by lesions of the medial prefrontal cortex (mPFC)48. The hippocampus has no major direct projections to the brainstem, but connects with NTS-projecting regions of the mPFC, such as the infralimbic cortex49, suggesting that hippocampal actions on autonomic function may be routed through the mPFC.
Medial prefrontal cortex (mPFC)
The mPFC is also complex, with different subregions contributing to different aspects of stress output (Figure 4). The prelimbic mPFC preferentially inhibits HPA-axis responses to psychogenic stressors50–53 and, like the hippocampus, regulates the duration but not the peak levels of glucocorticoid secretion, suggesting involvement in response termination. Inhibition of the prelimbic mPFC or local injection of noradrenaline enhances heart-rate responses to psychological stimuli54, consistent with a role for the prelimbic PFC in inhibiting autonomic stress responses.
In contrast, the infralimbic PFC is involved in the initiation of autonomic and HPA responses to psychogenic stimuli52, 55. Electrical stimulation of the ventromedial mPFC (which encompasses the infralimbic cortex) increases blood pressure in unanesthetized rats, whereas lesion or inactivation of this region inhibits conditioned cardiovascular responses56, 57. Inactivation of this area does not affect baseline heart rate or blood pressure, suggesting that the infralimbic cortex is selectively involved in stress-induced cardiovascular regulation, perhaps via modification of the parasympathetic component of the baroreflex58. Collectively, the data indicate that the prelimbic and infralimbic cortices have different roles in the coordination of stress responses, with outflow from the dorsal mPFC and the prelimbic cortex conferring inhibition, and that from the infralimbic cortex conferring stimulation. By virtue of its interconnections with the hippocampus and amygdala, the prefrontal cortex is positioned at the top of the response initiation hierarchy, and may be a principal, but not sole, limbic coordinator of physiological reactivity.
Other Limbic Sites
There is also evidence for stress-regulatory roles of other limbic sites, including the lateral septum, supramammillary nucleus and anterior thalamus40, 59, 60. For example, the lateral septum inhibits HPA axis and autonomic responses to acute stressors59, a role it shares with its principal afferent source, the hippocampus.
Top-down integration of glucocorticoid negative feedback
The HPA axis is subject to feedback inhibition by its principal product, glucocorticoids (Text Box 2), which is mediated at least in part by limbic forebrain structures. Both glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) are abundantly expressed in the hippocampus and are likely co-localized in some neurons61. As MRs and GRs have different affinities for corticosterone (see Box 2), this renders the hippocampus responsive to both basal and stress-induced elevations of corticosterone62. GRs are also highly expressed in the mPFC, but the expression of MRs is more limited here, suggesting that the mPFC may be less responsive to low glucocorticoid levels. Implants of corticosteroids in the mPFC inhibit HPA-axis responses to restraint but not ether51, supporting a possible role in feedback inhibition of psychogenic responses. Given that both the hippocampus and mPFC attenuate stress-induced increases in heart rate and blood pressure, it is possible that one or both glucocorticoid receptors influence autonomic outflow in addition to HPA axis activity, but this possibility has yet to be investigated.
Box 2. Corticosteroid receptors and negative feedback.
Glucocorticoids have both genomic and nongenomic actions thoughout the body. Genomic actions occur following binding to glucocorticoid receptor (GR) and, in the case of some tissues, mineralocorticoid receptors (MR), which act as ligand-activated transcription factors to affect broad, long-latency and biologically long-acting changes in gene transcription62. The MR has a high affinity for endogenous glucocorticoids and is extensively bound even during the circadian nadir of corticosteroid secretion. The GR has a lower affinity and is extensively bound only at relatively high levels of corticosteroids, such as during stress responses121. The GR appears to be the primary mediator of ‘delayed’ glucocorticoid inhibition of stress responses. In contrast, non-genomic effects occur within minutes of glucocorticoid release and likely involve action at the target cell membrane. Non-genomic signaling accounts for ‘fast’ negative feedback inhibition of the HPA axis, which occurs within minutes of the rise in circulating glucocorticoids (far too fast to be mediated by genomic actions in any traditional sense). It is currently unclear whether this mechanism involves membrane actions of the known nuclear receptors (GR and MR), or a novel, as yet to be identified membrane corticosteroid receptor.
Forebrain GRs are involved in regulating both basal HPA tone and termination of the stress response. Mice with selective deletion of the GR in the cortex, hippocampus and BLA have elevated basal glucocorticoids and prolonged corticosterone responses to psychogenic but not systemic stress63, 64, and are resistant to the inhibitory effect of exogenous glucocorticoids on HPA-axis activation63. Overexpression of GR in forebrain leads to reduced peak ACTH and corticosterone levels following stress, suggesting that forebrain GR are sufficient to reduce stress reactivity65, 66. Forebrain MRs have a more subtle role in acute HPA-axis feedback regulation. Deletion of MRs in the limbic forebrain has no effect on basal HPA tone or the peak response to restraint (recovery was not assessed)67. Overexpression of MR produces a mild suppression of acute stress responding only in female mice68.
Collectively, the data are consistent with a limbic interface between glucocorticoid signals and feedback regulation of the HPA axis. The relative importance of limbic glucocorticoid signaling depends on the stress modality: it regulates HPA axis responses to psychogenic but not systemic stress and so provides a context-specific feedback signal that modifies HPA axis inhibition.
Middle management of stress responses
Limbic structures have little direct anatomical interaction with primary stress effector systems. Intervening synapses are required to relay information from the amygdala, hippocampus and mPFC to the primary stress effector neurons in the PVN, caudal medulla and spinal cord3 (Figure 4). In general, output from stress-excitatory structures (such as the CeA, MeA and infralimbic cortex) mixes with that of stress-inhibitory regions (hippocampus, prelimbic cortex)3, providing opportunity for local integration of limbic information prior to accessing primary stress effectors.
Most limbic-PVN connections relay through GABA-rich cell groups in the bed nucleus of the stria terminalis (BST) and hypothalamus3. These inhibitory relays provide transsynaptic inhibition, translating excitatory glutamatergic outflow from the hippocampus and prelimbic cortex into inhibition of the PVN3. Descending information from the amygdala uses many of the same relay sites, but here the upstream neurons in the CeA and MeA are GABAergic69. Thus, activation of the PVN by these amygdala structures likely results from disinhibition3. Some degree of bisynaptic integration of stress outflow traverses excitatory networks as well. For example, the CeA and IL project to the NTS, which in turn excites the HPA axis and ANS70, 71.
Bed nucleus of the stria terminalis (BST)
The BST has numerous subregions that differ markedly in their contributions to stress integration (Figures 3–4). Anteroventral subregions are important in HPA-axis excitation, as lesions there reduce HPA-axis responses and inhibit acute activation of PVN neurons following restraint72, 73 (Figure 4). The anterolateral BST contains PVN-projecting CRH neurons74,75, suggesting a mechanism for central excitatory actions of CRH on the HPA axis. In contrast, lesions of the posteromedial BST increase ACTH and corticosterone secretion, PVN Fos activation and PVN CRH mRNA expression72, 73, consistent with a loss of inhibitory input to the HPA axis (Figure 4). Tracing studies indicate that PVN-projecting neurons in this region are predominantly GABAergic76, suggesting that, in contrast to the anterolateral PVN, posterior regions inhibit HPA responses to stress.
The precise role of the BST in autonomic regulation remains to be defined. In anesthetized rats, chemical or electrical stimulation of the BST produces predominantly depressor actions, particularly when the stimulation is directed to the anteromedial subregion77–79. However, in awake animals, pharmacological activation of this region elicits a rapid pressor response, followed by bradycardia80, 81, whereas inactivation exacerbates restraint-induced increases in heart rate82. This indicates that BST signaling is necessary for inhibiting cardiovascular responses to stress. Modulation of heart rate by BST stimulation or inhibition seems to be mediated by the parasympathetic nervous system80, 81.
Hypothalamic nuclei
Several hypothalamic regions provide potential interaction between descending limbic input and homeostatic integration. Hypothalamic relays between limbic sites and HPA and ANS output neurons provide a means to gate responses to stressors with respect to ongoing physiological status.
The medial preoptic hypothalamus (mPOA) supplies GABAergic innervation to the PVN (figure 3). mPOA lesions enhance HPA-axis responses to stressors83 and local inactivation enhances ACTH release, both consistent with a loss of inhibitory input to the PVN84. Moreover, mPOA lesions block the excitatory effect of amygdala stimulation on corticosterone release85, suggesting that the amygdala is an upstream modulator of mPOA neurons controlling the HPA axis response. Indeed, the mPOA is a prominent target of projections from the hippocampus and medial amygdala and is important in the integration of gonadal steroid signals, body temperature and sleep86; it thus provides a site for interaction between limbic inputs and physiological regulatory processes.
Limbic efferents also innervate the mediobasal hypothalamus, including the arcuate nucleus87 (Figure 3), which is a key regulator of energy balance and uses the PVN as a down-stream effector to regulate feeding and energy expenditure (via neuropeptidergic and GABAergic projections)88. The net output from the arcuate to the PVN is complex, communicating both orexigenic (neuropeptide Y, Agouti-related peptide) and anorexic (e.g., alpha-MSH)88 signals. Notably, many of these peptides activate the HPA axis, implying that both positive and negative energy balance represent homeostatic stressors89.
Lateral hypothalamic neurons are activated by stressors40, positioning them to modulate autonomic and/or HPA tone. The lateral hypothalamus is targeted by hippocampal, prefrontal cortical and amygdalar efferents and is important in the coordination of ingestive behaviors86. Glutamatergic, GABAergic and peptidergic neurons are highly intermingled in this region, making it difficult to predict its net impact on stress responses90, 91.
The suprachiasmatic nucleus (SCN) has a substantial impact on basal HPA and autonomic tone and on responses to psychogenic stressors92–94. The SCN has few direct projections to the PVN, but heavily innervates the area surrounding the PVN95, where it can interface with input from limbic sites. The SCN is the primary coordinator of physiologic rhythms, and is thus positioned to modulate HPA axis output in conjunction with time-of-day cues.
Neural control of chronic stress responses
Chronic stress exposure physically alters the structure and function of brain regions involved in controlling HPA and autonomic responses to stress (see Table 1). In the hippocampus and prefrontal cortex, chronic restraint causes retraction of apical dendrites and reduces spine density in pyramidal cells96, 97. Conversely, increased dendritic branching is observed in the BLA98. Chronic stress also changes PVN function, including increased expression of CRH and vasopressin mRNA99, 100, reduced GR expression99, 100 and altered expression of numerous neurotransmitter receptor subunits101, 102. Finally, neurochemical changes are seen in numerous stress-regulatory pathways that project to the PVN, including increased glutamic acid decarboxylase expression (implying increased GABA levels) in the hypothalamus and the BST103.
Table 1.
Neuroplastic Responses to Chronic Stress
| Site | Chronic Stress Plasticity | Process Impacted |
|---|---|---|
| HPC | Dendritic atrophy ↓GR expression |
↓HPA Glucocorticoid feedback ↓Memory |
| mPFC | Dendritic atrophy ↓GR expression |
↓HPA Glucocorticoid feedback ↓Extinction, ↓Reward |
| CeA | ↑CRH expression ↑CRH release |
↑Autonomic and HPA excitability ↑Anxiety |
| BLA | ↑dendritic branching ↑stress excitability |
↑Autonomic and HPA excitability ↓Emotional memory, ↓Reward |
| PVT | ↑stress excitability | ↑HPA excitability (novel stress) ↓HPA excitability (familiar stress) |
| LC | ↑neurotransmitter/NP ↑stress excitability (mPFC) |
↑HPA excitability (novel stress) |
| PVN | ↑secretagogue synthesis ↑stress receptivity ↓GR expression |
↑stress excitability (novel stress) |
Sensitization of stress responses
Neurochemical evidence suggests that chronic stress enhances the excitability of the HPA and the sympatho-adrenomedullary systems. Facilitated ACTH and corticosterone responses to novel stressors occur after chronic drive by either homotypical or unpredictable stress regimens104, 105. This response facilitation occurs despite clear evidence for ongoing or cumulative elevation in glucocorticoid levels, implying that feedback efficacy is diminished and/or drive is increased.
Chronic stress can recruit pathways that are distinct from those involved in acute responses. For example, lesions of the paraventricular thalamus inhibit the development of chronic-stress induced facilitation of HPA-axis activation, but do not affect responses to acute stress106, indicating that this region is engaged during repeated, chronic stress exposure. In contrast, lesions of the hippocampus do not affect HPA-axis responses associated with chronic stress47, suggesting that its role in HPA-axis regulation is diminished upon repeated stress exposure. The BST seems to be differentially involved in acute and chronic-stress responses, as lesions of the anterolateral BST reduce HPA-axis responses to acute stress, but enhance chronic-stress-induced facilitation of HPA axis activation107.
Repeated exposure to cold enhances stress-induced noradrenaline release in the frontal cortex and sensitizes the firing rate of locus coeruleus neurons following a novel stressor108–110. Chronic cold stress also increases the sensitivity of the locus coeruleus to CRH, suggesting that it increases the responsiveness to a factor that is released during stress109. Finally, chronic stress increases the expression of tyrosine hydroxylase, the rate-limiting enzyme in noradrenaline synthesis, in the locus coeruleus, as well as that of co-localized neuropeptides (e.g., galanin), which is consistent with an enhanced capacity for noradrenaline release (111, 112 but see also113). The locus coeruleus does not have substantial projections to the PVN5, suggesting that contributions to HPA-axis regulation are mediated by upstream (e.g., hippocampus, mPFC and amygdala) or downstream (ventrolateral medulla, spinal cord) targets.
The CeA is implicated in chronic stress regulation by virtue of its sensitivity to corticosteroids. High levels of glucocorticoids increase CRH mRNA expression in the CeA114, an effect that is mimicked by some chronic stress regimens (e.g., chronic immobilization, chronic pain)115 but not others (e.g., chronic restraint, chronic unpredictable stress, repeated predator exposure)116. In sheep, repeated stress (dog exposure) causes CRH release in the CeA, which appears crucial for sensitization of both the CRH response in the PVN and cortisol release upon repeated exposure117. The amygdalar CRH response to repeated stress is blocked by pretreatment with a GR antagonist, implying that corticosteroids are required for the enhanced CRH release in the CeA during repeated stress117, 118.
Although glucocorticoid secretion could be important for stress sensitization of the amygdala, chronic stress produces marked reductions in glucocorticoid signaling in other brain regions. Numerous chronic-stress regimens cause down-regulation of GR (and to a lesser extent, MR) mRNA, binding and protein levels in the mPFC and the hippocampus99, 119–121. This down-regulation could be associated with a loss of glucocorticoid negative-feedback sensitivity, at least in terms of inhibiting the circadian rise in corticosterone levels120, 121. Thus, chronic stress seems to be sufficient to both dampen negative-feedback signaling by stress-inhibitory pathways and to enhance positive drive through regions such as the CeA, which, together or alone, could contribute to an enhanced drive of the PVN.
Sensitization of autonomic responses is observed in some chronic stress models. In rats, chronic mild stress produces exaggerated heart rate and pressor responses to a novel stressor122. However, the neural substrates of ANS sensitization are not known.
Habituation of stress responses
Response habituation has been observed upon repeated exposure to mild stressors. In this case, the magnitude of the response diminishes with each exposure, even as responses to novel stressors are facilitated104, 123. The decreased physiological responses are paralleled by a decrement in central stress-induced Fos activation124. The habituation process seems to involve MRs, as systemic treatment with MR antagonists reverses the reduced corticosterone responses to repeated restraint125. Lesions of the paraventricular thalamus inhibit habituation of corticosterone responses to repeated restraint126 (however, see127) without affecting acute responding. In combination with the effects of lesions of this region on facilitation106, the paraventricular thalamus seems to be important in transducing information regarding the chronicity of stress exposure. Notably, this region receives heavy projections from the ventral subiculum and the mPFC76, 128, 129 and projects heavily to the CeA130, 131, providing a possible intermediary relay between stress-inhibitory and stress-excitatory brain regions.
Habituation of autonomic responses is highly dependent on the stress regimen. In mice, chronic social stress causes limited habituation of heart-rate responses to attack in submissive, but not dominant mice132. Chronic stress produces long-term changes in autonomic function, including increased heart rate, decreased heart rate variability (rats)122 and decreased blood pressure variability (mice)133. As was the case for response facilitation, the neural mechanisms underlying habituation of autonomic changes by chronic stress have yet to be explored.
Memory, reward and stress responses
Emotional learning and memory
Interpretating the predictive significance of environmental stimuli is crucial for appropriate control of physiological responses to stressors: the response to stimuli associated with a highly salient negative (or positive) outcome should produce a physiological response that is appropriate for coping with those stimuli, whereas a response generated to innocuous stimuli would be inefficient and metabolically costly. Thus, certain memories encourage responses to situations that predict an adverse outcome and discourage responses to irrelevant or habituated stimuli.
Limbic sites that regulate HPA and ANS responses — including the amygdala, hippocampus and mPFC — are involved in the conditioning of behavioral responses to emotional stimuli (reviewed in134,135–137) and are thus also poised to mediate conditioning of HPA and ANS activity. In support of this, conditioned HPA activation after conditioned taste aversion (Text Box 3) is blocked by hippocampectomy138, and the expression of conditioned HPA responses after contextual or tone-footshock conditioning is blocked by CeA lesions139. Conditioned pressor and tachycardia responses to contextual footshock conditioning are also reduced by pretreatment of the ventral mPFC with CoCl2 (a nonselective synapse blocker), an NMDA receptor antagonist, or nNOS inhibitors57,140.
Box 3. Conditioning of stress responses.
During exposure to aversive or stress-invoking stimuli, animals learn to associate predictive cues, whether they are contextual (e.g. environmental conditions) or discrete stimuli (e.g. auditory tones) with the onset of stress exposure. During this conditioning process, the predictive stimuli (or conditioned stimuli, CS) become capable of eliciting conditioned responses of the hypothalamus-pituitary-adrenal (HPA) and/or autonomic nervous system responses, presumably in anticipation of the forthcoming stressor.
HPA axis
Some of the earliest work on conditioned activation of the HPA axis used a conditioned taste aversion paradigm. In this model, rats are exposed to a tastant (e.g. a saccharin drink) that is followed by administration of a drug that induces visceral illness (e.g. lithium chloride). When these rats are subsequently given the tastant again, there is a conditioned increase in plasma corticosterone154, 155. Conditioned HPA activation is also observed with the conditioned fear paradigm, in which rats receive a footshock in a unique environment (contextual conditioning) or are given a discrete signal (tone or light) paired with a footshock. Upon re-exposure to either the context or the cue there is a conditioned increase in plasma corticosterone139.
Autonomic nervous system
Conditioned autonomic responses also occur, however the particular nature of the conditioned response varies with the experimental paradigm. Conditioned increases in heart rate, blood pressure, plasma catecholamines (i.e. adrenaline and noradrenaline), and/or sympathetic nerve activity have been shown to occur upon re-exposure to a tone previously paired with shock receipt142, 156, 157, the unique environment after contextual footshock conditioning158–160, a non-electrified probe after previously receiving shock from the probe160, 161, a cage after contextual airjet stress conditioning162, and a tone predicting tail pinch or shock163.
There is also evidence for conditioning of stress responses at the level of the relay areas to HPA and autonomic effectors. Lesions of the BST block corticosterone responses to contextual, but not tone, conditioning, purportedly due to connections between the BST and hippocampus139. Lesions of the perifornical hypothalamus and lateral hypothalamus disrupt some types of conditioned stress responses141–144, raising the possibility that conditioned HPA and ANS activation might also be integrated at the level of limbic-hypothalamic relay areas, which in turn suggests that both limbic and homeostatic signals are involved in the generation of learned responses.
Stress and reward
There seems to be a reciprocal relationship between reward and stress processing in the brain. Exposure to natural rewards buffers the effect of stressors on HPA activity, and stress increases reward-seeking behavior (e.g., intake of palatable food, reinstatement of drug-taking behavior). Experiences with rewarding stimuli generally evoke stress-like HPA and ANS responses (see Text Box 4). The effects vary depending on whether the reward is ‘natural’ (e.g., sexual behavior, voluntary wheel running, palatable food intake) or ‘pharmacological’ (e.g., drugs of abuse), and on whether the experiences were self-administered (e.g, contingent on level pressing, self-choice) or investigator-delivered.
Box 4. Reward and stress effectors.
Acute exposure to rewarding stimuli
When naïve rats are first given acute noncontingent administration of rewarding drugs (i.e. drugs of abuse) there typically is a robust activation of the HPA axis and an increase in sympatho-vagal activity (reflected by increased heart rate, blood pressure and/or circulating catecholamines) (cocaine164–166, amphetamine164, 167, 168, nicotine169, 170, morphine171, 172, ethanol173–177). Many of these drugs can have direct actions, both in the periphery and at central sites (for example, activation of the reward system), that can contribute to altered activity of the hypothalamus-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS)178, 179. In addition, contingent (i.e. self) administration of drugs can reduce HPA and ANS responses169,176, 180, 181. Importantly, acute experiences with naturally rewarding stimuli do not always evoke stress-like responses182–185,186, 187.
Chronic exposure to rewarding stimuli
Chronic drug exposure generally induces a chronic stress-like state in which basal tone of the HPA axis and ANS and/or their responses to stress are facilitated188–191,192–196. The effects of chronic exposure to natural rewards have been little investigated, with the exception of chronic intake of palatable foods: in no-choice paradigms (e.g. rats given sucrose drink instead of water, or lard mixed with chow), chronic intake of palatable food generally increases sympathetic and/or HPA axis tone197,198,199,200–203. In contrast, in choice paradigms (e.g. rats are offered sucrose drink and/or lard in addition to water and chow), chronic intake of palatable food reduces HPA and sympathetic activation after stress105, 202, 204–206. Moreover, the artificial sweetener saccharin reproduces some of these effects105, 206, suggesting that palatability or reward, rather than the calories contained in the food, is sufficient to evoke these responses. Thus, the nature of the effect of repeated exposure to natural rewards on the HPA axis and ANS seems to depend largely on whether the rats chose to engage in the natural reward.
Conditioned responses to rewarding stimuli
Cues that predict exposure to drugs of abuse elicit conditioned activation of the HPA axis and ANS207, 208. However, when the appetitive stimuli are natural rewards the conditioned response is more complex; cues generally evoke anticipatory HPA axis and ANS activation with a rapid inactivation upon receipt of the reward209–211. Moreover, if the natural reward is not provided as expected, then there is a further increase in HPA axis activation (“frustration”)209–213.
The primary brain reward circuit consists of dopaminergic projections from the ventral tegmental area to the nucleus accumbens (NAc) and has extensive connections with the BLA and mPFC (reviewed in145–147). The NAc, BLA and mPFC are widely recognized as key mediators of responses to natural rewards and drugs of abuse145–147. As the mPFC and BLA regulate stress responses and are involved in conditioning to salient emotional stimuli, these structures are likely to regulate HPA and ANS responses to both unconditioned and conditioned reward exposure. It is not clear whether the NAc also regulates HPA axis and ANS activity, but projections from the NAc core and shell to brain regions such as the lateral hypothalamus, BST, lateral preoptic area and parabrachial nucleus148, 149 provide an anatomical substrate for potential physiological effects on the HPA axis and ANS. Thus, there is considerable anatomical overlap among the brain regions subserving reward processing, emotional learning and stress responses.
Overlapping circuits mediating stress, memory and reward: functional significance
Stress responses allow the animal to cope with aversive stimuli that represent real or anticipated threats to homeostasis, but mounting a stress response is energetically demanding. Thus, there is an advantage to learning when a particular situation is not a threat and to preemptively reducing an ensuing stress response; moreover, it is advantageous to be able to associate specific environmental cues with a particular stressor so that stress responses can be mounted in anticipation of predicted stressors, thereby minimizing homeostatic disruption.
Rewarding stimuli (particularly those that are novel, unpredicted and/or not self-administered) can be considered stressful because they disrupt homeostasis. In particular, drugs of abuse elicit strong unconditioned and conditioned activation of the HPA axis and ANS that are linked to the addiction process150, 151. However, rewarding stimuli provide fundamentally different experiences from other types of stressors because animals are motivated to obtain them. In fact, chronic voluntary engagement in naturally rewarding behaviors, such as palatable food intake, reduces stress responses; these fundamentally ‘pleasurable’ experiences seem to have anti-stress effects. In this manner, the brain can tailor stress responses based on the collective experiences of an individual.
Conclusions and future directions
The neural control of stress is a complex process that requires integration of information regarding both real and potential outcomes. Higher-order processing of stress involves weighing the potential value of a given stimulus against potential negative outcomes. The polysynaptic organization of stress pathways further suggests that inputs relaying information on the physiological status of the animal can contribute to the eventual endocrine or autonomic response to the stressor. The vast majority of ‘decisions’ regarding the initiation of physiological stress responses seem to be made at the level of limbic structures, which communicate information to subcortical sites positioned to interface with ongoing homeostatic feedback.
Emerging functional studies suggest a considerable division of labor among limbic sites. Connections between the infralimbic cortex, CeA, ventrolateral BST, PVN and NTS with autonomic effectors suggest a means by which the important immediate effector — the sympatho-adrenomedullary system — is brought on line. Responses of the other effector — the HPA axis — involve a network that connects the prelimbic cortex, MeA, hippocampus, posterior BST, preoptic area and other hypothalamic nuclei with hypophysiotrophic neurons of the PVN; these connections serve to initiate as well as terminate glucocorticoid release in response to neural and hormonal feedback. The physical separation of autonomic and HPA stress effector circuits promotes some degree of independence of the two stress-modulatory cascades, allowing for appropriate tuning of neural and hormonal responses to specific demand characteristics of the real or anticipated event. However, these two physiological systems also work together, both in terms of overlap in their underlying neural circuitry and their physiological functions. Future growth of the stress physiology field will need to include more work that crosses over the proverbial HPA vs. ANS divide, thereby providing a more complete picture of how an individual learns to anticipate and otherwise cope with stress.
Whereas the effector circuits that control stress are likely hard-wired, the overall weighting of information is subject to considerable individual variation. It is likely that dysfunctions of information processing across these circuits, as a result of environmental adversity and/or genetic factors, lie at the root of maladaptive stress reactions that can culminate in affective disease (e.g., depression, post-traumatic stress disorder) and physical infirmities. Importantly, stress research generally focuses on the use of ‘negative’ stimuli as stressors (i.e., situations that individuals would avoid if possible). However, ‘positive’ stimuli (such as novel rewards) can cause comparable physiological stress responses despite the fact that individuals are motivated to obtain them. An understanding of how and why this apparent contradiction occurs is necessary to appreciate why individuals make the choices that they do, and how the brain and body cope with the consequences of their actions.
Acknowledgments
The authors thank present and past members of the Herman lab for their contributions to this work, and Dr. Stephen Woods for his comments on the review. The authors’ work cited in this review was supported by NIH grants MH049698, MH069680, MH069725, AG12962 and DK078906.
Glossary
- Bradycardic response
slowing of heart rate
- Brainstem
portion of brain including the pons, medulla and midbrain
- Chronic stress
Ongoing or repeated exposure to one or more types of stress stimuli over a period ranging from days to months
- Depressor response
decrease in blood pressure
- Effector
final common pathway controlling autonomic or neuroendocrine responses
- Fos
immediate early gene product whose expression is tightly linked to recent cellular excitation
- Glutamic Acid Decarboxylase (GAD)
enzyme responsible of synthesis of GABA. Exists in two isoforms (GAD65, GAD67)
- Limbic System
Collection of highly-interconnected forebrain structures involved in processing emotion and memory
- Postganglionic
Second neuron in the two-neuron chain mediating autonomic responses
- Preautonomic neurons
CNS neurons involved in regulation of preganglionic sympathetic or parasympathetic neurons in the spinal cord and/or brainstem
- Preganglionic
First neuron in the two-neuron chain mediating autonomic responses
- Pressor response
increase in blood pressure
- Subfornical organ
forebrain blood-brain barrier deficient circumventricular organ
- Visceral afferent
ascending sensory nerves from thoracic and abdominal organs
Bibliography
- 1.Iversen S, Iversen L, Saper CB. In: Principles of Neural Science. Kandel ER, Schwartz JH, Jessell TM, editors. Mc-Graw Hill; New York: 2000. [Google Scholar]
- 2.Droste SK, et al. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–53. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- 3.Herman JP, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–67. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 6.Plotsky PM, Cunningham ET, Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocrine Rev. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- 7.Hokfelt T, et al. Coexistence of peptides with classical transmitters. Experientia. 1987;43:768–780. doi: 10.1007/BF01945354. [DOI] [PubMed] [Google Scholar]
- 8.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–70. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 9.Sawchenko PE, Arias C, Bittencourt JC. Inhibin beta, somatostatin and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1990;291:269–280. doi: 10.1002/cne.902910209. [DOI] [PubMed] [Google Scholar]
- 10.Buller K, Xu Y, Dayas C, Day T. Dorsal and ventral medullary catecholamine cell groups contribute differentially to systemic interleukin-1beta-induced hypothalamic pituitary adrenal axis responses. Neuroendocrinology. 2001;73:129–38. doi: 10.1159/000054629. [DOI] [PubMed] [Google Scholar]
- 11.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–67. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- 12.Kinzig KP, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–70. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–23. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI. J Neurosci. 2002;22:9635–42. doi: 10.1523/JNEUROSCI.22-21-09635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–60. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- 16.Krause EG, et al. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–24. doi: 10.1210/en.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plotsky PM, Sutton SW, Bruhn TO, Ferguson AV. Analysis of the role of angiotensin II in mediation of adrenocorticotropin secretion. Endocrinology. 1988;122:538–545. doi: 10.1210/endo-122-2-538. [DOI] [PubMed] [Google Scholar]
- 18.Swanson LW, Lind RW. Neural projections subserving the initiation of a specific motivated behavior in the rat: new projections from the subfornical organ. Brain Res. 1986;379:399–403. doi: 10.1016/0006-8993(86)90799-7. [DOI] [PubMed] [Google Scholar]
- 19.Johnson AK, Cunningham JT, Thunhorst RL. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol. 1996;23:183–91. doi: 10.1111/j.1440-1681.1996.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 20.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–70. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 21.Kreier F, et al. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–7. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- 22.Decavel C, Van Den Pol AN. GABA: A dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 23.Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–69. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol. 2007;582:539–51. doi: 10.1113/jphysiol.2007.133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus of the rat. J Comp Neurol. 1993;332:123–143. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 26.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–5. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 27.Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R8–15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- 28.Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996;16:1173–9. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curran T, et al. Inducible proto-oncogene transcription factors: third messengers in the brain? Cold Spring Harb Symp Quant Biol. 1990;55:225–34. doi: 10.1101/sqb.1990.055.01.024. [DOI] [PubMed] [Google Scholar]
- 30.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–80. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 31.Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 33.Sawchenko PE, et al. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–222. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- 34.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Day TA, Buller KM. The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1beta administration. Neuroscience. 1999;94:175–83. doi: 10.1016/s0306-4522(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 36.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–22. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 37.Prewitt CM, Herman JP. Hypothalamo-Pituitary-Adrenocortical regulation following lesions of the central nucleus of the amygdala. Stress. 1997;1:263–280. doi: 10.3109/10253899709013746. [DOI] [PubMed] [Google Scholar]
- 38.Roozendaal B, Koolhaas JM, Bohus B. Differential effect of lesioning of the central amygdala on the bradycardiac and behavioral response of the rat in relation to conditioned social and solitary stress. Behav Brain Res. 1990;41:39–48. doi: 10.1016/0166-4328(90)90052-g. [DOI] [PubMed] [Google Scholar]
- 39.Roozendaal B, Koolhaas JM, Bohus B. Central amygdala lesions affect behavioral and autonomic balance during stress in rats. Physiol Behav. 1991;50:777–81. doi: 10.1016/0031-9384(91)90017-i. [DOI] [PubMed] [Google Scholar]
- 40.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 41.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 42.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–9. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- 43.Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15:173–85. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson L, Sapolsky RM. The role of the hippocampus in feedback regulation of the hypothalamo-pituitary-adrenocortical axis. Endocrine Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 45.Rubin RT, Mandell AJ, Crandall PH. Corticosteroid responses to limbic stimulation in man: localization of stimulation sites. Science. 1966;153:1212–1215. doi: 10.1126/science.153.3737.767. [DOI] [PubMed] [Google Scholar]
- 46.Dunn JD, Orr SE. Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res. 1984;54:1–6. doi: 10.1007/BF00235813. [DOI] [PubMed] [Google Scholar]
- 47.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 48.Ruit KG, Neafsey EJ. Cardiovascular and respiratory responses to electrical and chemical stimulation of the hippocampus in anesthetized and awake rats. Brain Res. 1988;457:310–21. doi: 10.1016/0006-8993(88)90701-9. [DOI] [PubMed] [Google Scholar]
- 49.Ruit KG, Neafsey EJ. Hippocampal input to a “visceral motor” corticobulbar pathway: an anatomical and electrophysiological study in the rat. Exp Brain Res. 1990;82:606–16. doi: 10.1007/BF00228802. [DOI] [PubMed] [Google Scholar]
- 50.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–64. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 51.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamo-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–76. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerrits M, et al. Increased stress vulnerability after a prefrontal cortex lesion in female rats. Brain Res Bull. 2003;61:627–35. doi: 10.1016/j.brainresbull.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol. 2001;13:625–37. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan RM, Gratton A. Behavioral effects of excitotoxic lesions of ventral medial prefrontal cortex in the rat are hemisphere-dependent. Brain Res. 2002;927:69–79. doi: 10.1016/s0006-8993(01)03328-5. [DOI] [PubMed] [Google Scholar]
- 56.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–93. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 57.Resstel LB, Joca SR, Guimaraes FG, Correa FM. Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience. 2006;143:377–85. doi: 10.1016/j.neuroscience.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Resstel LB, Fernandes KB, Correa FM. Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Res. 2004;1015:136–44. doi: 10.1016/j.brainres.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 59.Dobrakovova M, Kvetnansky R, Torda T, Murgas K. Changes of plasma and adrenal catecholamines and corticosterone in stressed rats with septal lesions. Physiol Behav. 1982;29:41–5. doi: 10.1016/0031-9384(82)90363-8. [DOI] [PubMed] [Google Scholar]
- 60.Suarez M, Maglianesi MA, Perassi NI. Involvement of the anterodorsal thalami nuclei on the hypophysoadrenal response to chronic stress in rats. Physiol Behav. 1998;64:111–6. doi: 10.1016/s0031-9384(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 61.Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res. 2005;51:371–81. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 62.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 63.Boyle MP, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–8. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–90. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei Q, et al. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci. 2007;27:8836–44. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei Q, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–6. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berger S, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci U S A. 2007;104:4688–93. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–31. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 70.Schwaber JS, Kapp BS, Higgins GA, PR R. Amygdala and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- 72.Choi DC, et al. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray TS, et al. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- 74.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 75.Champagne D, Beaulieu J, Drolet G. CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: a tract-tracing study. J Neuroendocrinol. 1998;10:119–31. doi: 10.1046/j.1365-2826.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- 76.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: Evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 77.Ciriello J, Janssen SA. Effect of glutamate stimulation of bed nucleus of the stria terminalis on arterial pressure and heart rate. Am J Physiol. 1993;265:H1516–22. doi: 10.1152/ajpheart.1993.265.5.H1516. [DOI] [PubMed] [Google Scholar]
- 78.Gelsema AJ, Calaresu FR. Chemical microstimulation of the septal area lowers arterial pressure in the rat. Am J Physiol. 1987;252:R760–7. doi: 10.1152/ajpregu.1987.252.4.R760. [DOI] [PubMed] [Google Scholar]
- 79.Hatam M, Nasimi A. Glutamatergic systems in the bed nucleus of the stria terminalis, effects on cardiovascular system. Exp Brain Res. 2007;178:394–401. doi: 10.1007/s00221-006-0748-4. [DOI] [PubMed] [Google Scholar]
- 80.Alves FH, Crestani CC, Resstel LB, Correa FM. Cardiovascular effects of carbachol microinjected into the bed nucleus of the stria terminalis of the rat brain. Brain Res. 2007;1143:161–8. doi: 10.1016/j.brainres.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 81.Crestani CC, Alves FH, Resstel LB, Correa FM. Both alpha1 and alpha2-adrenoceptors mediate the cardiovascular responses to noradrenaline microinjected into the bed nucleus of the stria terminal of rats. Br J Pharmacol. 2008;153:583–90. doi: 10.1038/sj.bjp.0707591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crestani CC, Alves FH, Tavares RF, Correa FM. Role of the bed nucleus of the stria terminalis in the cardiovascular responses to acute restraint stress in rats. Stress. 2008 doi: 10.1080/10253890802331477. in press. [DOI] [PubMed] [Google Scholar]
- 83.Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamo-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett. 2006;397:291–6. doi: 10.1016/j.neulet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 85.Feldman S, Conforti N, Saphier D. The preoptic area and bed nucleus of the stria terminalis are involved in the effects of the amygdala on adrenocortical secretion. Neuroscience. 1990;37:775–9. doi: 10.1016/0306-4522(90)90107-f. [DOI] [PubMed] [Google Scholar]
- 86.Swanson LW. In: Handbook of Chemical Neuroanatomy. Björklund A, Hökfelt T, Swanson LW, editors. Elsevier; Amsterdam: 1987. pp. 1–124. [Google Scholar]
- 87.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–45. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- 88.Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol. 1998;402:435–41. [PubMed] [Google Scholar]
- 89.Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 90.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2005;387:80–4. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 91.Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–29. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- 92.Cascio CS, Shinsako J, Dallman MF. The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Res. 1987;423:173–178. doi: 10.1016/0006-8993(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 93.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–13. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buijs RM, Wortel J, Van Heerikhuize JJ, Kalsbeek A. Novel environment induced inhibition of corticosterone secretion: physiological evidence for a suprachiasmatic nucleus mediated neuronal hypothalamo-adrenal cortex pathway. Brain Res. 1997;758:229–36. doi: 10.1016/s0006-8993(97)00234-5. [DOI] [PubMed] [Google Scholar]
- 95.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: Studies using anterograde transport of phaseolus vulgaris-leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 96.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 97.Radley JJ, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–50. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herman JP, Adams D, Prewitt CM. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 100.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 101.Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol. 2000;419:344–51. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 102.Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol. 2005;484:43–56. doi: 10.1002/cne.20445. [DOI] [PubMed] [Google Scholar]
- 103.Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci. 1998;18:5938–47. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akana SF, et al. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131:57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 105.Ulrich-Lai YM, et al. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–34. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary- adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 107.Choi DC, et al. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149:818–26. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nisenbaum LK, Abercrombie ED. Presynaptic alterations associated with enhancement of evoked release and synthesis of norepinephrine in hippocampus of chronically cold-stressed rats. Brain Res. 1993;608:280–7. doi: 10.1016/0006-8993(93)91469-9. [DOI] [PubMed] [Google Scholar]
- 109.Jedema HP, Finlay JM, Sved AF, Grace AA. Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol Psychiatry. 2001;49:351–9. doi: 10.1016/s0006-3223(00)01057-x. [DOI] [PubMed] [Google Scholar]
- 110.Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81:1055–64. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- 111.Holmes PV, Blanchard DC, Blanchard RJ, Brady LS, Crawley JN. Chronic social stress increases levels of preprogalanin mRNA in the rat locus coeruleus. Pharmacol Biochem Behav. 1995;50:655–60. doi: 10.1016/0091-3057(94)00334-3. [DOI] [PubMed] [Google Scholar]
- 112.Watanabe Y, et al. Effects of chronic social stress on tyrosine hydroxylase mRNA and protein levels. Brain Res Mol Brain Res. 1995;32:176–80. doi: 10.1016/0169-328x(95)00081-3. [DOI] [PubMed] [Google Scholar]
- 113.Smith MA, Brady LS, Glowa J, Gold PW, Herkenham M. Effects of stress and adrenalectomy on tyrosine hydroxylase mRNA levels in locus coeruleus by in situ hybridization. Brain Res. 1991;544:26–32. doi: 10.1016/0006-8993(91)90881-u. [DOI] [PubMed] [Google Scholar]
- 114.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 115.Ulrich-Lai YM, et al. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav. 2006;88:67–76. doi: 10.1016/j.physbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 116.Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–58. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 117.Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–62. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 118.Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav. 2002;75:455–64. doi: 10.1016/s0031-9384(02)00650-9. [DOI] [PubMed] [Google Scholar]
- 119.Gomez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–37. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 120.Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–97. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 121.Reul JM, deKloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 122.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–41. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 123.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress–comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–8. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 124.Stamp J, Herbert J. Corticosterone modulates autonomic responses and adaptation of central immediate-early gene expression to repeated restraint stress. Neuroscience. 2001;107:465–79. doi: 10.1016/s0306-4522(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 125.Cole MA, et al. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol. 2000;12:1034–42. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- 126.Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–10. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- 127.Fernandes GA, et al. Habituation and cross-sensitization of stress-induced hypothalamic-pituitary-adrenal activity: effect of lesions in the paraventricular nucleus of the thalamus or bed nuclei of the stria terminalis. J Neuroendocrinol. 2002;14:593–602. doi: 10.1046/j.1365-2826.2002.00819.x. [DOI] [PubMed] [Google Scholar]
- 128.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–76. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 129.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 130.Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–87. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- 131.Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–38. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- 132.Bartolomucci A, et al. Chronic psychosocial stress persistently alters autonomic function and physical activity in mice. Physiol Behav. 2003;80:57–67. doi: 10.1016/s0031-9384(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 133.Farah VM, Joaquim LF, Bernatova I, Morris M. Acute and chronic stress influence blood pressure variability in mice. Physiol Behav. 2004;83:135–42. doi: 10.1016/j.physbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 134.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 135.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 136.Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–23. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- 137.Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smotherman WP, Kolp LA, Coyle S, Levine S. Hippocampal lesion effects on conditioned taste aversion and pituitary-adrenal activity in rats. Behav Brain Res. 1981;2:33–48. doi: 10.1016/0166-4328(81)90037-1. [DOI] [PubMed] [Google Scholar]
- 139.Sullivan GM, et al. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 140.Resstel LB, de Aguiar Correa FM, Silveira Guimaraes F. The Expression of Contextual Fear Conditioning Involves Activation of an NMDA Receptor Nitric Oxide Pathway in the Medial Prefrontal Cortex. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kosten T, Contreras RJ. Deficits in conditioned heart rate and taste aversion in area postrema-lesioned rats. Behav Brain Res. 1989;35:9–21. doi: 10.1016/s0166-4328(89)80003-8. [DOI] [PubMed] [Google Scholar]
- 142.Supple WF, Jr, Leaton RN. Lesions of the cerebellar vermis and cerebellar hemispheres: effects on heart rate conditioning in rats. Behav Neurosci. 1990;104:934–47. doi: 10.1037//0735-7044.104.6.934. [DOI] [PubMed] [Google Scholar]
- 143.Furlong T, Carrive P. Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res. 2007;1128:107–19. doi: 10.1016/j.brainres.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 144.Iwata J, LeDoux JE, Reis DJ. Destruction of intrinsic neurons in the lateral hypothalamus disrupts the classical conditioning of autonomic but not behavioral emotional responses in the rat. Brain Res. 1986;368:161–6. doi: 10.1016/0006-8993(86)91055-3. [DOI] [PubMed] [Google Scholar]
- 145.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 146.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 147.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 148.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 149.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 150.Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–9. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- 151.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cannon WB. Bodily changes in pain, hunger, fear and rage; an account of recent researches into the function of emotional excitement. D. Appleton and Company; New York, London: 1929. [Google Scholar]
- 153.Ulrich-Lai YM, Engeland WC. Sympatho-adrenal activity and hypothalamic-pituitary-adrenal axis regulation. In: Steckler T, Kalin NH, Reul J, editors. Handbook of Stress and the Brain. Elsevier B.V.; Amsterdam: 2005. pp. 419–435. [Google Scholar]
- 154.Ader R. Conditioned adrenocortical steroid elevations in the rat. J Comp Physiol Psychol. 1976;90:1156–63. doi: 10.1037/h0077290. [DOI] [PubMed] [Google Scholar]
- 155.Smotherman WP, Hennessy JW, Levine S. Plasma corticosterone levels as an index of the strength of illness induced taste aversions. Physiol Behav. 1976;17:903–8. doi: 10.1016/0031-9384(76)90006-8. [DOI] [PubMed] [Google Scholar]
- 156.Iwata J, LeDoux JE. Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat. Behav Neurosci. 1988;102:66–76. doi: 10.1037//0735-7044.102.1.66. [DOI] [PubMed] [Google Scholar]
- 157.Randall DC, Brown DR, Brown LV, Kilgore JM. Sympathetic nervous activity and arterial blood pressure control in conscious rat during rest and behavioral stress. Am J Physiol. 1994;267:R1241–9. doi: 10.1152/ajpregu.1994.267.5.R1241. [DOI] [PubMed] [Google Scholar]
- 158.Nijsen MJ, et al. Conditioned fear-induced tachycardia in the rat: vagal involvement. Eur J Pharmacol. 1998;350:211–22. doi: 10.1016/s0014-2999(98)00261-1. [DOI] [PubMed] [Google Scholar]
- 159.Carrive P. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Res. 2000;858:440–5. doi: 10.1016/s0006-8993(00)02029-1. [DOI] [PubMed] [Google Scholar]
- 160.Korte SM, et al. Conditioned neuroendocrine and cardiovascular stress responsiveness accompanying behavioral passivity and activity in aged and in young rats. Physiol Behav. 1992;51:815–22. doi: 10.1016/0031-9384(92)90120-q. [DOI] [PubMed] [Google Scholar]
- 161.Diamant M, Croiset G, De Zwart N, De Wied D. Shock-prod burying test in rats: autonomic and behavioral responses. Physiol Behav. 1991;50:23–31. doi: 10.1016/0031-9384(91)90493-8. [DOI] [PubMed] [Google Scholar]
- 162.Inagaki H, Kuwahara M, Tsubone H. Effects of psychological stress on autonomic control of heart in rats. Exp Anim. 2004;53:373–8. doi: 10.1538/expanim.53.373. [DOI] [PubMed] [Google Scholar]
- 163.Nakamura K, Ono T, Fukuda M, Uwano T. Paraventricular neuron chemosensitivity and activity related to blood pressure control in emotional behavior. J Neurophysiol. 1992;67:255–64. doi: 10.1152/jn.1992.67.2.255. [DOI] [PubMed] [Google Scholar]
- 164.Budziszewska B, Jaworska-Feil L, Lason W. The effect of repeated amphetamine and cocaine administration on adrenal, gonadal and thyroid hormone levels in the rat plasma. Exp Clin Endocrinol Diabetes. 1996;104:334–8. doi: 10.1055/s-0029-1211463. [DOI] [PubMed] [Google Scholar]
- 165.Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–6. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- 166.Saphier D, Welch JE, Farrar GE, Goeders NE. Effects of intracerebroventricular and intrahypothalamic cocaine administration on adrenocortical secretion. Neuroendocrinology. 1993;57:54–62. doi: 10.1159/000126342. [DOI] [PubMed] [Google Scholar]
- 167.Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav. 1993;45:629–37. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- 168.Knych ET, Eisenberg RM. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology. 1979;29:110–8. doi: 10.1159/000122912. [DOI] [PubMed] [Google Scholar]
- 169.Donny EC, et al. Differential effects of response-contingent and response-independent nicotine in rats. Eur J Pharmacol. 2000;402:231–40. doi: 10.1016/s0014-2999(00)00532-x. [DOI] [PubMed] [Google Scholar]
- 170.Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther. 1987;243:217–26. [PubMed] [Google Scholar]
- 171.Nikolarakis KE, Pfeiffer A, Stalla GK, Herz A. Facilitation of ACTH secretion by morphine is mediated by activation of CRF releasing neurons and sympathetic neuronal pathways. Brain Res. 1989;498:385–8. doi: 10.1016/0006-8993(89)91122-0. [DOI] [PubMed] [Google Scholar]
- 172.Zubelewicz B, Muc-Wierzgon M, Harbuz MS, Brodziak A. Central single and chronic administration of morphine stimulates corticosterone and interleukin (IL)-6 in adjuvant-induced arthritis. J Physiol Pharmacol. 2000;51:897–906. [PubMed] [Google Scholar]
- 173.Patel VA, Pohorecky LA. Acute and chronic ethanol treatment on beta-endorphin and catecholamine levels. Alcohol. 1989;6:59–63. doi: 10.1016/0741-8329(89)90074-8. [DOI] [PubMed] [Google Scholar]
- 174.Guaza C, Torrellas A, Borrell S. Adrenocortical response to acute and chronic ethanol administration in rats. Psychopharmacology (Berl) 1983;79:173–6. doi: 10.1007/BF00427806. [DOI] [PubMed] [Google Scholar]
- 175.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–31. [PubMed] [Google Scholar]
- 176.Koranyi L, Endroczi E, Tal E, Levay G. The effect of acute and chronic ethanol administration on serum corticosterone concentration in rats. Acta Physiol Hung. 1987;69:123–8. [PubMed] [Google Scholar]
- 177.Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52:481–9. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- 178.Tella SR, Schindler CW, Goldberg SR. Cocaine: cardiovascular effects in relation to inhibition of peripheral neuronal monoamine uptake and central stimulation of the sympathoadrenal system. J Pharmacol Exp Ther. 1993;267:153–62. [PubMed] [Google Scholar]
- 179.Mo W, Arruda JA, Dunea G, Singh AK. Cocaine-induced hypertension: role of the peripheral sympathetic system. Pharmacol Res. 1999;40:139–45. doi: 10.1006/phrs.1999.0503. [DOI] [PubMed] [Google Scholar]
- 180.Terry LC, Martin JB. Hypothalamic-pituitary responses to intracranial self-stimulation in the rat. Brain Res. 1978;157:89–104. doi: 10.1016/0006-8993(78)90998-8. [DOI] [PubMed] [Google Scholar]
- 181.Burgess ML, et al. Effects of intracranial self-stimulation on selected physiological variables in rats. Am J Physiol. 1993;264:R149–55. doi: 10.1152/ajpregu.1993.264.1.R149. [DOI] [PubMed] [Google Scholar]
- 182.Retana-Marquez S, Bonilla-Jaime H, Velazquez-Moctezuma J. Lack of effect of corticosterone administration on male sexual behavior of rats. Physiol Behav. 1998;63:367–70. doi: 10.1016/s0031-9384(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 183.Bonilla-Jaime H, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S. Hormonal responses to different sexually related conditions in male rats. Horm Behav. 2006;49:376–82. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 184.Szechtman H, Lambrou PJ, Caggiula AR, Redgate ES. Plasma corticosterone levels during sexual behavior in male rats. Horm Behav. 1974;5:191–200. doi: 10.1016/0018-506x(74)90043-9. [DOI] [PubMed] [Google Scholar]
- 185.Bronson FH, Rissman EF. Epinephrine release in response to sexual activity in male versus female rats. Physiol Behav. 1989;45:185–9. doi: 10.1016/0031-9384(89)90183-2. [DOI] [PubMed] [Google Scholar]
- 186.Yancey SL, Overton JM. Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol. 1993;75:1334–40. doi: 10.1152/jappl.1993.75.3.1334. [DOI] [PubMed] [Google Scholar]
- 187.Niijima A. Effects of taste stimulation on the efferent activity of the autonomic nerves in the rat. Brain Res Bull. 1991;26:165–7. doi: 10.1016/0361-9230(91)90203-v. [DOI] [PubMed] [Google Scholar]
- 188.Houshyar H, Gomez F, Manalo S, Bhargava A, Dallman MF. Intermittent morphine administration induces dependence and is a chronic stressor in rats. Neuropsychopharmacology. 2003;28:1960–72. doi: 10.1038/sj.npp.1300271. [DOI] [PubMed] [Google Scholar]
- 189.Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent d-amphetamine induces sensitization of HPA axis to a subsequent stressor. Neuropsychopharmacology. 2002;26:286–94. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- 190.Borowsky B, Kuhn CM. Chronic cocaine administration sensitizes behavioral but not neuroendocrine responses. Brain Res. 1991;543:301–6. doi: 10.1016/0006-8993(91)90041-s. [DOI] [PubMed] [Google Scholar]
- 191.Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, ‘binge’ cocaine administration: diminished individual differences in stress-induced corticosterone response. Neuroendocrinology. 1998;68:334–44. doi: 10.1159/000054382. [DOI] [PubMed] [Google Scholar]
- 192.Mantsch JR, et al. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett. 2007;415:269–73. doi: 10.1016/j.neulet.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mantsch JR, et al. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–11. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patterson-Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R. Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol. 2005;37:157–66. doi: 10.1016/j.alcohol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 195.Lee S, Rivier C. Effect of exposure to an alcohol diet for 10 days on the ability of interleukin-1 beta to release ACTH and corticosterone in the adult ovariectomized female rat. Alcohol Clin Exp Res. 1993;17:1009–13. doi: 10.1111/j.1530-0277.1993.tb05656.x. [DOI] [PubMed] [Google Scholar]
- 196.Macho L, Zorad S, Radikova Z, Patterson-Buckedahl P, Kvetnansky R. Ethanol consumption affects stress response and insulin binding in tissues of rats. Endocr Regul. 2003;37:195–202. [PubMed] [Google Scholar]
- 197.Bunag RD, Tomita T, Sasaki S. Chronic sucrose ingestion induces mild hypertension and tachycardia in rats. Hypertension. 1983;5:218–25. doi: 10.1161/01.hyp.5.2.218. [DOI] [PubMed] [Google Scholar]
- 198.LeBlanc J, Labrie A. A possible role for palatability of the food in diet-induced thermogenesis. Int J Obes Relat Metab Disord. 1997;21:1100–3. doi: 10.1038/sj.ijo.0800520. [DOI] [PubMed] [Google Scholar]
- 199.Walgren MC, Young JB, Kaufman LN, Landsberg L. The effects of various carbohydrates on sympathetic activity in heart and interscapular brown adipose tissue of the rat. Metabolism. 1987;36:585–94. doi: 10.1016/0026-0495(87)90172-7. [DOI] [PubMed] [Google Scholar]
- 200.Balkan B, Strubbe JH, Bruggink JE, Steffens AB. Overfeeding-induced obesity in rats: insulin sensitivity and autonomic regulation of metabolism. Metabolism. 1993;42:1509–18. doi: 10.1016/0026-0495(93)90144-d. [DOI] [PubMed] [Google Scholar]
- 201.Kamara K, Eskay R, Castonguay T. High-fat diets and stress responsivity. Physiol Behav. 1998;64:1–6. doi: 10.1016/s0031-9384(97)00534-9. [DOI] [PubMed] [Google Scholar]
- 202.la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–9. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 203.Tannenbaum BM, et al. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol. 1997;273:E1168–77. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- 204.Bell ME, et al. Sucrose intake and corticosterone interact with cold to modulate ingestive behaviour, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. J Neuroendocrinol. 2002;14:330–42. doi: 10.1046/j.1365-2826.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- 205.Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272:R840–8. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- 206.Suchecki D, Antunes J, Tufik S. Palatable solutions during paradoxical sleep deprivation: reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect on energy imbalance. J Neuroendocrinol. 2003;15:815–21. doi: 10.1046/j.1365-2826.2003.01067.x. [DOI] [PubMed] [Google Scholar]
- 207.Ettenberg A, McFarland K. Effects of haloperidol on cue-induced autonomic and behavioral indices of heroin reward and motivation. Psychopharmacology (Berl) 2003;168:139–45. doi: 10.1007/s00213-002-1266-0. [DOI] [PubMed] [Google Scholar]
- 208.DeVries AC, Taymans SE, Sundstrom JM, Pert A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res. 1998;786:39–46. doi: 10.1016/s0006-8993(97)01328-0. [DOI] [PubMed] [Google Scholar]
- 209.Coe CL, Stanton ME, Levine S. Adrenal responses to reinforcement and extinction: role of expectancy versus instrumental responding. Behav Neurosci. 1983;97:654–7. doi: 10.1037//0735-7044.97.4.654. [DOI] [PubMed] [Google Scholar]
- 210.de Boer SF, de Beun R, Slangen JL, van der Gugten J. Dynamics of plasma catecholamine and corticosterone concentrations during reinforced and extinguished operant behavior in rats. Physiol Behav. 1990;47:691–8. doi: 10.1016/0031-9384(90)90079-j. [DOI] [PubMed] [Google Scholar]
- 211.Romero LM, Levine S, Sapolsky RM. Adrenocorticotropin secretagog release: stimulation by frustration and paradoxically by reward presentation. Brain Res. 1995;676:151–6. doi: 10.1016/0006-8993(95)00111-3. [DOI] [PubMed] [Google Scholar]
- 212.Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kawasaki K, Iwasaki T. Corticosterone levels during extinction of runway response in rats. Life Sci. 1997;61:1721–8. doi: 10.1016/s0024-3205(97)00778-9. [DOI] [PubMed] [Google Scholar]



