Preface
Brain function relies on the ability of neurons to communicate with each other. Interneuronal communication primarily takes place at synapses, where information from one neuron is rapidly conveyed to a second neuron. There are two main modalities of synaptic transmission: chemical and electrical. Far from functioning independently and serving unrelated functions, mounting evidence indicates that these two modalities of synaptic transmission closely interact, both during development and in the adult brain. Rather than conceiving synaptic transmission as either chemical or electrical, this article emphasizes the notion that synaptic transmission is both chemical and electrical and that interactions between these two forms of interneuronal communication might be required for normal brain development and function.
Keywords: Gap junction, Connexin, innexin, Glutamate, Mixed synapse, Development, Synaptic plasticity, Brain injury
Introduction
Communication between neurons is required for brain function and the quality of such communication is thought to underlie dynamic aspects of hardwired neural networks. Functional interactions between neurons occur at anatomically identifiable cellular regions called synapses. Although the nature of synaptic transmission has been an area of enormous controversy (See Box 1), two main modalities of synaptic transmission are now recognised: chemical and electrical. At chemical synapses, the information is transferred via the release of a neurotransmitter from one cell that is detected by an adjacent cell1, whereas in the in the case of electrical synapses the cytoplasm of adjacent cells are directly connected by clusters of intercellular channels called gap junctions2. While these specializations for both forms of transmission can be found at various neuronal sites (dendrites, somata, axons), chemical transmission normally occurs between synaptic terminals along axons and the dendrite or soma of a second neuron, a muscle fiber or gland cell. The presence of these two modalities of synaptic transmission does not exclude the possibility that brain cells could also communicate via alternative mechanisms, such as volume transmission (diffusion through the extracellular space of neurotransmitters that reach remote target cells)3 and by generating electrical fields that are capable of influencing the excitability of nearby neurons4.
Box 1. Communication between neurons: the debate over the nature of synaptic transmission.
Santiago Ramón y Cajal and Charles Sherrington, the fathers of modern neuroscience, established that networks of multiple elementary units or “neurons”, communicate with each other via functional specializations called “synapses”. Their seminal contributions were followed by a bitter debate over the nature of synaptic transmission; was it mediated by chemical or electrical signals? This controversy was known as the war of “Soup vs. Sparks”166. Although several researchers, most notably T.R. Elliott167 and later Otto Loewi168 demonstrated the existence of neurotransmitters with actions on postsynaptic cells, there was still controversy over whether transmitter release could occur in a fraction of a millisecond, the synaptic “delay” indirectly measured by Sherrington169. Bernard Katz and colleagues demonstrated that synaptic transmission at the frog neuromuscular junction was an electrically mediated, Ca++-dependent form of transmitter release, which occurs within a fraction of a millisecond170. Such mechanism was showed to also occur in the central nervous system, leading to a general agreement that synaptic transmission was chemically mediated. But in 1958, David Potter communicated at a “Monday night fight” of the Marine Biological Laboratory in Woods Hole (so called because of the contentious nature of the scientific exchanges) the striking properties of synaptic transmission in crayfish, which challenged all the criteria established for chemical transmission. Postsynaptic signals reproduced the time course of presynaptic signals, transmission was bidirectional and, surprisingly, voltage-dependent. The finding provided the earliest evidence in support for the existence of electrical synaptic transmission171 and was soon followed by seminal studies in teleost brain by Michael V.L. Bennett and colleagues172 and David Robertson173 and Edwin Furshpan174, in which physiological and ultrastructural analysis were combined. Their search for the anatomical basis of electrical transmission greatly contributed to identifying the cellular structures that we know today as gap junctions. The more recent demonstration of the ubiquitous presence of electrical synapses in the mammalian brain led to the indisputable conclusion that chemical and electrical transmissions co-exist in all nervous systems.
Electrical and chemical synapses are known to coexist in most organisms and brain structures, but details of the properties and distribution of these two modalities of transmission are still emerging. Most research efforts have focused on exploring the mechanisms of chemical transmission, and significantly less is known about those underlying electrical transmission. It was thought that electrical synapses were more abundant in invertebrates and cold-blooded vertebrates and less prevalent in mammals. However, a wealth of data now indicates a widespread distribution of electrical synapses in the mammalian brain5. In addition to the retina, inferior olive and olfactory bulb, structures where electrical transmission was known to occur5, electrical synapses have been found in disparate regions of the mammalian central nervous system and shown to constitute a distinct phenotypic feature of inhibitory interneurons in general6–8.
Perhaps because the appreciation of each form of synaptic transmission occurred in a sequential manner (at least in mammals), there is a widespread notion that electrical and chemical synapses operate independently. This article will review the evidence that chemical and electrical synapses functionally interact during development and in adulthood. Rather than being a comprehensive review of the experimental evidence, here we focus on a few examples that highlight the ability of chemical and electrical synapses to interact with each other.
Are chemical synapses more sophisticated, than electrical synapses?
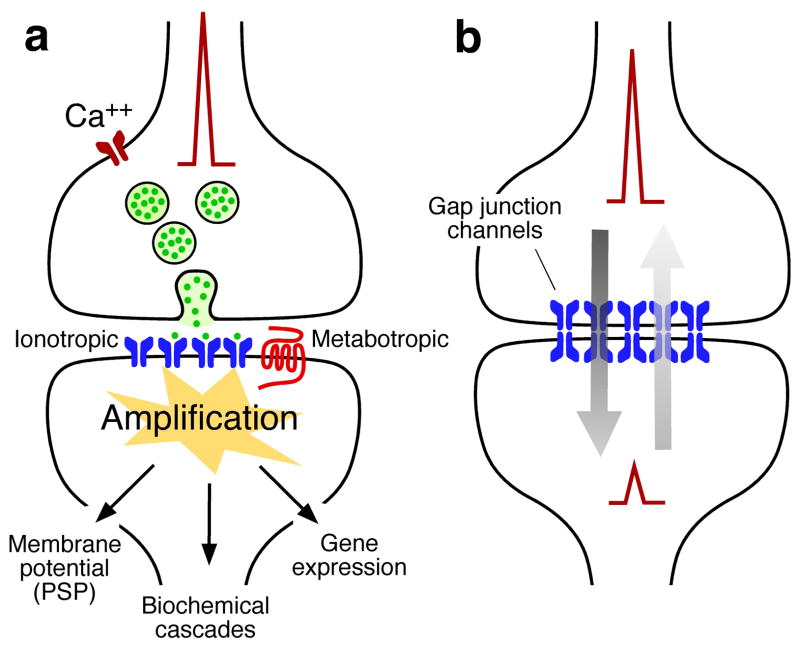
From the evolutionary point of view, chemical communication between cells preceded electrical communication9. Communication with chemical signals occurs in unicellular organisms such as bacteria10, mediating important phenomena such as quorum sensing11 (the mechanism underlying detection of bacterial population density). More specific communication between individual cells requires cellular specializations between the interacting partners, a characteristic that neuronal chemical and electrical synapses inshare with immunological synapses between a lymphocyte and antigen-presenting cell.12 From this point of view, chemical transmission requires sophisticated presynaptic molecular machinery that regulates neurotransmitter release in a probabilistic manner when an action potential invades the synaptic terminal1 (although at some contacts, release can also be proportional to changes in resting membrane potential)13. A similarly complex postsynaptic molecular machinery is required, including inotropic (ligand-gated ion channels) and metabotropic receptors (G-protein coupled receptors that act indirectly through a secondary messenger) (Fig. 1a), that are capable of detecting and translating this message into various postsynaptic events, ranging from changes in resting potential (‘synaptic potential’) to gene expression1. These features allow chemical synapses to adapt to diverse functional requirements. The mechanisms and general properties of chemical synaptic transmission have been extensively reviewed [see Ref. 1 for review].
Figure 1. The two main modalities of synaptic transmission.
a, Chemical transmission requires sophisticated presynaptic molecular machinery that regulates neurotransmitter release in a probabilistic manner upon depolarization of the presynaptic terminal, in this case by the arrival of an action potential. A similarly complex postsynaptic molecular machinery is also required. This includes the presence of inotropic and metabotropic receptors that are capable of detecting and translating the presynaptic message (neurotransmitter) into various postsynaptic events, ranging from changes in resting potential to gene expression. b, Electrical transmission is mediated by clusters of intercellular channels called gap junctions that communicate the interior of two adjacent cells, directly allowing the bi-directional passage of electrical currents carried by ions (arrows), as well intracellular messengers and small metabolites (not illustrated here). Electrical synapses are bi-directional in nature: when a “presynaptic” action potential propagates to the “postsynaptic” cell, the membrane resting potential of the “postsynaptic” cell simultaneously propagates to the “presynaptic” cell (arrows).
Electrical transmission is mediated by gap junctions between neurons2, clusters of intercellular channels that directly communicate the interiors of two adjacent cells (Fig. 1b), directly allowing the bi-directional passage of electrical currents and small molecules (Ca++, cAMP, IP3, amongst others). Gap junction channels are formed by the docking of two hexameric connexin ‘hemichannels’ or ‘connexons’ contributed by each of the adjacent cells14. Strikingly, although they assemble into almost identical structures connexons in invertebrates and vertebrates are formed by two different multigene families of proteins: connexins, which are unique to chordates, and innexins and pannexins, which are unique to invertebrates, prechordates and some chordates2,14. In contrast to innexins, pannexins do not seem to form intercellular channels in tissues and thus are thought to operate only as unpaired hemichannels in vertebrates15. Such evolutionary convergence highlights the functional relevance of gap junction intercellular communication. From a family of over 20 genes in mammals14, only a handful of connexins were found to be expressed in vertebrate neurons [See Ref. 15 for review]16. Because of its widespread distribution, connexin 36 (Cx36) is considered to be the main ‘synaptic’ connexin17. Several of the 25 members of the innexin family have been identified in invertebrate neurons, mainly in fly18, leech19 and worm20.
Although electrical synapses can act, to some extent, in a metabotropic fashion by allowing the passage of small metabolites, they lack the ability to amplify and transform presynaptic signals, as chemical synapses do. However, this does not mean that electrical synapses are less sophisticated; their sophistication just relies on a different functional property, their bi-directionality, which allows them to coordinate the activity of large groups of interconnected neurons2. Based on this bi-directional and analogic (that is, they don’t require an action potential) nature, electrical synapses are especially efficient in detecting the coincidence of simultaneous subthreshold depolarizations within a group of coupled neurons, a phenomenon that increases neuronal excitability and promotes synchronous firing21–25. Electrical synapses are also very effective at mediating lateral excitation and increasing the sensitivity of sensory systems, such of those observed in retina26 and primary afferents of escape networks27–29. Gap junction-mediated lateral excitation has also been shown to occur in a network of cerebellar interneurons, promoting the spread of chemically mediated synaptic inputs between remote dendritic arborizations30. Because of their reliability (transmission at electrical synapses is not probabilistic in nature) and absence of synaptic delay (transmission occurs instantaneously), electrical synapses are also a usual feature in escape response networks, in both invertebrates29,31,32 and vertebrates33. Thus, from the functional point of view, no particular form of transmission is ‘better’ than the other one. Rather, their individual mechanisms seem to be specifically adapted to rescue and communicate different aspects of cellular processing and function.
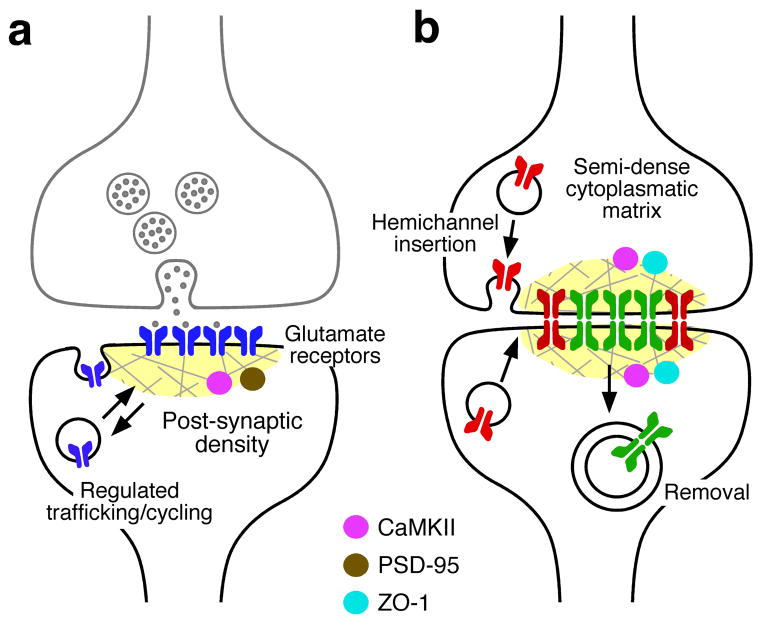
Bolstered by the implicit molecular complexity of chemical transmission and its ability to undergo plasticity, there was until recently the widespread notion that electrical transmission is a rather simple, static and rigid form of neuronal communication. However, increasing evidence suggests that electrical synapses are structurally more complex and functionally more dynamic than previously anticipated. Far from being static, gap junctions were found to be very dynamic structures when recombinant connexins were expressed in cell expression systems and the turnover of gap junction proteins was monitored with imaging techniques34–39. Recent data indicate that native neuronal gap junctions are also very dynamic structures and that their channels are actively turned over40. More specifically, ultrastructural and functional analysis at identified mixed (electrical and chemical) synapses on the goldfish Mauthner cell (a large reticulospinal neuron in fish)33,41 revealed that gap junction hemichannels are simultaneously added at the edges of gap junction plaques (clusters of intercellular gap junction channels) where they dock with hemichannels in the apposed membranes to form cell-cell channels and that intact junctional regions are removed from the centre of these plaques into either presynaptic axon or the postsynaptic Mauthner cell dendrite40. Moreover, electrical coupling was readily modified by peptides that interfere with endocytosis or exocytosis, suggesting that the strength of electrical synapses at these terminals is determined, at least in part, by a fast turnover of gap junction channels40. The half-life of this turnover was estimated to be 1–3 hours40, a time consistent with that observed for other ion channels and synaptic receptors42 indicating that this dynamic process could be involved in regulating electrical transmission (Fig. 2). Thus, as in glutamatergic chemical synapses43–47, regulated trafficking of connexons and intercellular channels could underlie modifications of gap junctional conductance. Furthermore, like their chemical counterparts, electrical synapses are highly modifiable by the action of neuromodulators such as dopamine and capable of activity-dependent plasticity [See Ref. 15 for review]16.
Figure 2. Trafficking of channels at chemical and electrical synapses.
a, Glutamate receptors are trafficked in and out of synapses. Postsynaptic densities provide a scaffold that helps to regulation this trafficking. PSD-95 and CaMKII are both abundant components of postsynaptic densities. Regulated trafficking of AMPA receptors (blue) is thought to underlie the modification of synaptic strength at glutamatergic synapses. b, Gap junction channels at electrical synapses turnover. New connexons are trafficked to the membrane in vesicles as unpaired hemichannels, where they are inserted at the periphery of the gap junction plaque and dock with hemichannels in the apposed membrane. They are internalized as small clusters of entire channels (green) into either of the coupled cells from regions near the center of the plaque. Proteins in the “semi dense cytoplasmatic matrix” act as scaffold. ZO-1 is a structural component whereas CaMKII seems to be a non-obligatory component of the macromolecular complex with functions that might be similar to those at postsynaptic densities of chemical synapses.
The existence of such dynamic structural and functional properties indicates that electrical transmission must rely on more than the intercellular channels. Constitutive and regulated trafficking of ligand-gated ion channels to and from the plasma membrane are important processes for the maintenance of chemical synaptic function. At glutamatergic synapses this requires interactions between the receptors’ carboxy-terminals and various cytosolic proteins and scaffolding proteins46,48,49, mostly located at the postsynaptic density (PSD)50 (Fig. 2a). Similarly, electrical synapses require the molecular machinery to mediate the turnover of gap junction channels. Gap junctions are now considered to be part of multiprotein complexes51–53. Electron dense areas similar to those of PSDs, referred as “semi-dense cytoplasmic matrix”53, have been observed at neuronal gap junctions when explored by electron microscopy (Fig. 2b). The detailed composition of these PSD-like structures is largely unknown54, although several molecules are known to interact with Cx3655–58 and its teleost homologs59, most notably zonula occludens one (ZO-1)56,57,59 and Ca++/calmodulin-dependent kinase II (CaMKII)51,52. ZO-1 is a scaffold protein of the MAGUK family that is known to associate to many connexins60, and that might have a similar role to PSD-95 at glutamatergic synapses1. Conserved regions of the carboxy-terminus of Cx36 (and its two teleost homologs)58,59,61 mediate interactions with ZO-1 and are required for the insertion of new gap junction proteins into electrical synapses40,61. ZO-1 has been also proposed to have an essential role regulating the transition from connexon to intercellular gap junction channel formation in the periphery of Cx43-containing junctions62. In contrast to the permanent structural role of ZO-1, CaMKII has been proposed to be a non-obligatory component of electrical synapses and its association linked to synaptic activity (see below)52. Such regulatory associated proteins seem to be crucial for innexin-based electrical transmission as well, as the stomatin-like protein (unc-1) was shown to be required for proper channel function at gap junctions formed by UNC-9 innexin in C. elegans63,64.
Recent data suggests that, similar to pre- and postsynaptic sites at chemical synapses, one side of an electrical synapse should not necessarily be considered to be the mirror image of the other65. Molecular asymmetries in neuronal gap junctions suggest that electrical synapses are functionally diverse. The intercellular channels at electrical synapses can in some cases be constructed by apposition of two different connexins, forming “heterotypic” (as opposed to “homotypic”) gap junctions14. Gap junctions at auditory mixed synapses of the goldfish Mauthner cell are constructed by apposition of hemichannels formed by two homologs of mammalian Cx3665; while Cx35 is restricted to presynaptic hemiplaques, Cx34.7 it was found only in postsynaptic hemiplaques, forming heterotypic junctions65. Heterotypic gap junction channels have been associated with rectification of electrical transmission65–68, defined as the propensity of some electrical synapses to display differential resistance to current flow in one vs. the other direction across the junction. Thus, such molecular asymmetry can support rectification of electrical transmission which, by favoring the spread of membrane responses from the Mauthner cell to presynaptic endings, promotes cooperativity between auditory afferents. This association was initially observed in invertebrates66 and is also supported by a wealth of work on heterotypic channels formed by recombinant connexins in cell expression systems67–69. Although asymmetry based on the presence of two Cx36 homologs is restricted to teleost fish (teleost fish underwent an additional genome duplication)70, the finding provided unambiguous evidence that vertebrate electrical synapses can be asymmetric, and that such asymmetry might underlie important functional properties. Molecular asymmetry in electrical synapses might not be restricted to the existence of molecularly different connexons but could also result from posttranslational modifications of individual connexins or differences in the complement of gap junction-associated proteins forming the “semi-dense cytoplasmatic matrix”54, endowing electrical transmission with more complex properties.
In summary, although chemical synapses seem structurally more complex and functionally dynamic, emerging evidence indicates that electrical synapses might be similarly complex, diverse and highly modifiable.
Electrical and chemical synapses interact during development
Gap junctional communication between neurons is developmentally regulated: it is initially prominent but declines at later developmental stages71,72. In the mammalian brain, this form of intercellular communication is widespread between postnatal days 5 and 12 and dramatically decreases after postnatal day 16 to remain restricted to some cell types71,73–76. The initial increase in coupling allows developing neurons to form functional domains that exhibit coordinated patterns of spontaneous activity74,77,78. It has been proposed that the formation of these transient domains and networks might serve as a developmental blueprint, influencing a variety of developmental cellular phenomena, ranging from cell contact inhibition, neuronal differentiation, migration, circuit formation and elimination of chemical synapses72.
The formation of these transient gap junction networks is a common feature of invertebrate79–82 and vertebrate71,72,75,76 nervous systems. In c. elegans, a transient network formed by the innexin gap junction protein NSY-5 is required for the left and right olfactory AWC neurons to create asymmetric patterns of gene expression during embryogenesis82. NSY-5 mutants failed to establish this asymmetry, suggesting that intercellular communication mediated by NSY-5 junctions is a critical developmental requirement82. Similarly, transient gap junctions mediate avoidance of segmental homolog cell projections during development in the leech83. Upon contact, the processes from AP neurons, two identifiable homolog cells, stop growing and finally retract, thus defining the topography of their neuronal arbors84–86. Recent data suggest that transient innexin 1 (INX-1) - containing gap junctions that are formed between processes of homolog AP cells mediate this developmental phenomenon83. Neurite growth arrest did not occur in animals in which INX-1 was knocked down by expressing a short hair-pin interfering RNA, nor on those expressing a INX-1 mutant transgene lacking open channel pore function but that retains the adhesive cellular binding of gap junction channels83. These findings suggest that gap junctions serve as conduits for an unknown messenger molecule that mediates the effect. Another well-established example of transient gap junction network occurs in the developing mammalian spinal cord87–89. Developing lumbar motorneurons are communicated by gap junctions, leading to synchronous patterns of nerve activity during a period in which muscle fibers are innervated by axons originating from multiple motorneurons87,89. The transition from multiple innervation to the characteristic single innervation in muscle fibers occurs at late embryonic and early postnatal stages and is an activity-dependent process, which results from competition between active synapses90. It was shown that a transient gap-junction network modulates this developmental transition from multiple to single fiber innervation91. Gap junctional coupling between motorneurons disappears at late embryonic and early postnatal stages causing lack of correlated activity between innervating axons (elimination cannot occur if synapses are synchronously activated), which in turn paves the way for synapse competition and subsequent synapse elimination in multiply innervated muscle fibers91. Reduction of gap junctional communication with gap junction blocking agents disrupted synchronous patterns of nerve activity and enhanced synapse elimination91. Consistent with this finding, acceleration of synapse elimination was observed in Cx40 KO mice92, a connexin that is expressed in developing motorneurons and that dramatically decreases after birth87, indicating a primary role for transient gap junction networks in gating the transition from multiple to single fiber muscle innervation.
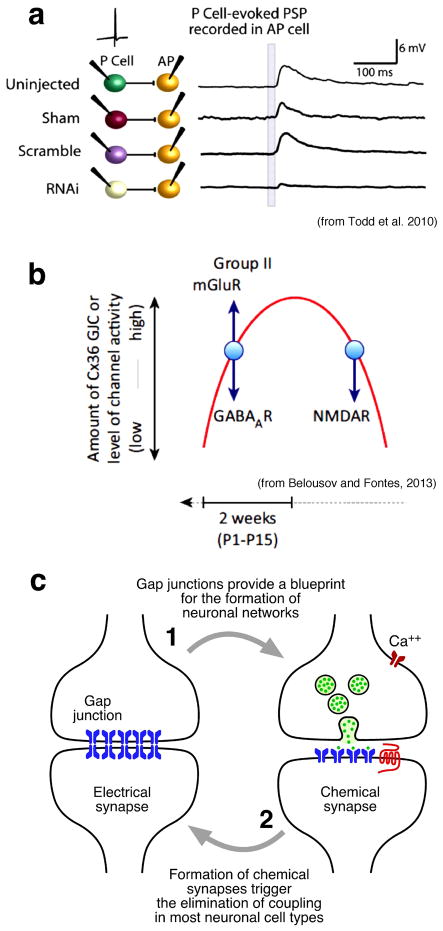
Remarkably, the formation and subsequent elimination of gap junction coupling between neurons was found to be correlated with the emergence of chemical neurotransmission in several species. This robust phenomenon was again observed at developing invertebrate79,93,94 and vertebrate nervous systems95–97. Furthermore, several studies indicate that gap junction proteins expressed during development are required for the formation of chemical synapses. Flies containing mutations in the innexin genes Shaking B and Ogre failed to establish appropriate functional synaptic connections in the visual system of this organism, as evidenced by the absence or alterations in the electroretinogram98. This developmental defect could be rescued by transgenic expression of the lost innexins during pulpal development, but not at later stages98. More direct evidence of the relationship between electrical synapses and the formation of chemical synapses was observed at identifiable motorneurons of the snail Helisoma93. Manipulations that led to a reduction of coupling between these snail motorneurons accelerated the formation of chemical synapses, whereas, exposure to cholinergic antagonists (which block chemical neurotransmission at these synapses) caused prolonged maintenance of electrical transmission93, suggesting an inverse relationship between the establishment of chemical and electrical connectivity at early stages of synaptic development. Such relationship between the formation of electrical and chemical synapses was also observed in vertebrates. The presence of gap junction coupling between developing spinal motorneurons also followed an inverse relationship with the formation of chemical synapses95. The decline in motorneuron coupling that occurs at late embryonic and early postnatal age is correlated with the formation of glutamatergic synapses in these spinal circuits95. Furthermore, exposure to NMDA receptor antagonists arrested the developmental decline in gap junction coupling between spinal motorneurons, suggesting that the increase in glutamatergic synaptic activity that is generally associated with the onset of locomotion promotes the disappearance of gap junction-mediated networks between developing motorneurons95. The exquisite relationship between the early presence of electrical connections and the subsequent formation of chemical synapses has recently been demonstrated in the leech. The sequential development of electrical and chemical synapses between two types of identifiable neurons that participate in a behaviorally relevant local-bending circuit offered the possibility of directly addressing this issue79. Synaptic transmission between the P cell (that transduces pressure) and AP cell (a motorneuron-like cell) is initially exclusively electrical and becomes predominantly chemical in the adult animal. Although electrical transmission remains, it is considerably weakened94. Silencing INX-1 in embryonic P cells when innexin-based gap junctions are initially forming and chemical synapses are absent (~50% embryonic development), prevented not only the establishment of electrical synapses but also the development of chemical transmission94 (Fig. 3a), elegantly demonstrating the requirement of electrical synapses for the formation of chemical contacts. Moreover, the development of networks mediated by chemical synapses in the mouse olfactory bulb was arrested in Cx36 knockout mice96 in which electrical transmission is greatly reduced, indicating that the early presence of electrical synapses is also a prerequisite for the formation of chemical synapses in mammals. This phenomenon was also observed in the developing neocortex. Consecutive divisions of individual radial glial progenitor cells normally produce sister excitatory neurons that lead to the formation of ontogenetic cortical columns that preferentially develop specific chemical synapses with each other rather than with nearby non-siblings97. Sister excitatory neurons are initially electrically coupled97 and blockade of this communication impairs the subsequent formation of specific chemical synapses between sister excitatory neurons in ontogenetic cortical columns97.
Figure 3. Electrical and chemical synapses interact during development.
a, Blockade of electrical synapse formation in leech embryos perturbs the formation of chemical synapses. Chemical synaptic potentials in an AP cell in response to a single spike in a P cell (marked by the gray bar on the AP recordings) under control conditions (uninjected, sham, scrambled) and after injection of double-stranded RNA (RNAi) that interferes with the translation of INX-1 in embryonic P cells, at a developmental time (~50% embryonic development) at which innexin-based gap junctions are forming but chemical synapses have not yet formed. [Data from Todd et al., 2010 (figure will be redrawn by the journal’s graphic art department).] b, Effect of signaling through GABAA receptor (GABAAR), metabotropic glutamate receptor mGluR) and NMDA receptor (NMDAR) on gap junction communication during development. Red line represents the increase (upward phase) and decrease (downward phase) in the amount of neuronal gap junction communication (GJC) and expression of connexin 36 (Cx36) during development. Blue arrows show the direction of the change in gap junction communication after activation of the receptor. P1 and P15 indicate postnatal days 1 and 15. [Taken from Belousov and Fontes et al., 2013 (figure will be redrawn by the journal’s graphic art department)] c, Modalities of interactions between electrical and chemical synapses during development.
We have discussed the mechanistic relationship between the presence of electrical synapses and the subsequent formation of chemical connections. However, conversely, receptors for chemical neurotransmitters and chemical synapses have been shown to regulate the emergence and disappearance of gap junctions during development99–101. In-vitro studies of cortical and hypothalamic neurons indicate that, despite that chemical connections are not yet formed, activation of specific receptors for chemical neurotransmitters regulate the expression of Cx36 at early developmental stages100. Prolonged (two weeks)100 activation of group II metabotropic glutamate receptors (mGluRs) leads to an increase in the expression of Cx36 via a cAMP/PKA dependent intracellular pathway, whereas activation of type A GABA receptors (GABAARs) leads to a reduction of Cx36 expression100 (Fig. 3b), indicating that the formation of gap junctional networks relies on the interplay between these two types of receptors. Because GABAARs are depolarizing at these early developmental stages, their effects are mediated via Ca++ influx and activation of PKC100. This developmental regulation of Cx36 gene expression involves regulation of both gene transcription (mGluR-dependent increase) and protein translation (GABAAR-dependent decrease)100. These changes are thought to be specific for these receptors100.
Activation of glutamate receptors also reduces coupling observed at late developmental stages and as mentioned blocking NMDA receptors delayed the formation of chemical synapses in spinal motorneurons95, suggesting that the emergence of glutamatergic transmission regulates uncoupling. This is supported by findings in the rat hypothalamus99. Activation of NMDA receptors at late developmental stages led to Ca++/cAMP response element binding protein (CREB)-dependent down regulation of Cx36 gene expression99, providing a direct mechanistic relationship between the emergence of chemical transmission and the disappearance of neuronal gap junction coupling (Fig. 3b).
In summary, the development of neural circuits in disparate nervous systems seems to critically rely on interactions between chemical and electrical synapses, which reciprocally and dynamically regulate the emergence of these two forms of transmission (Fig. 3c).
Electrical and chemical synapses interact in the adult nervous system
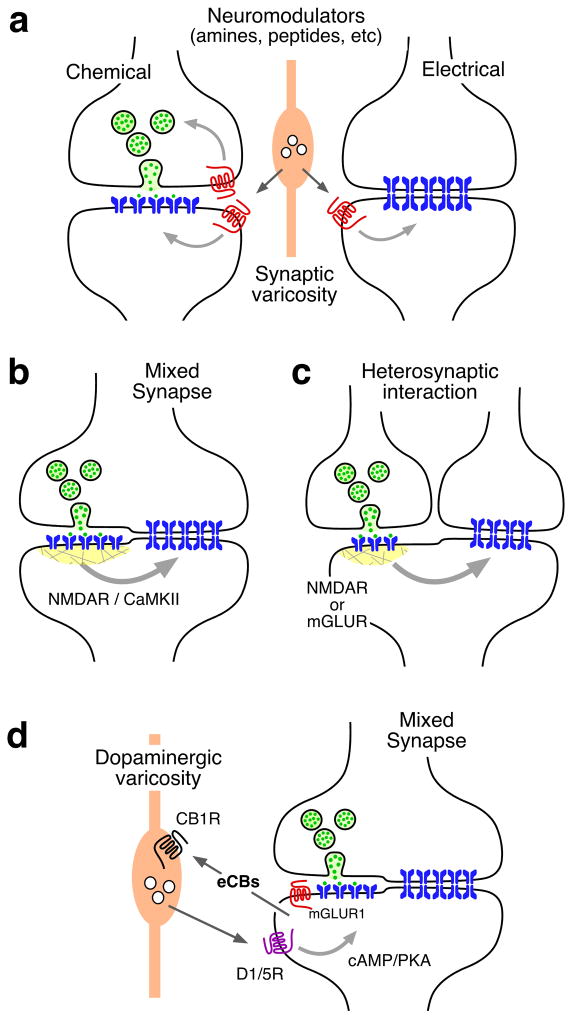
The effects of a special type of neurotransmitters: neuromodulators on gap junction communication represent a clear example of the interactions between chemical and electrical transmission in the adult nervous system. Neuromodulators are released from presynaptic terminals and activate specific metabotropic G-protein coupled receptors which trigger signaling cascades that modulate synaptic communication between target cells (Fig. 4a). Dopamine for example, has been shown to modulate gap junction communication at various vertebrate and invertebrate cell types [See Refs. 16 and 102 for review]16,102, suggesting that both connexin and innexin gap junction proteins are targets of its regulatory actions. At electrical synapses mediated by Cx36 or Cx57, and its teleost homologs, activation of dopamine can lead to either an increase or a decrease of gap junctional conductance between neurons via a cAMP-dependent mechanism that involves the activation of PKA. That is, dopamine was observed to enhance coupling at goldfish electrical synapses103,104 and to promote decoupling of between retinal horizontal cells105,106,107. Two phosphorylation sites in Cx36 and its fish homologs, Ser110 and Ser293 (Ser276 in teleost)107,108, seem to be essential for the effects of dopamine on electrical synapses. Recent evidence indicates that dopamine acting via D1/5 receptors, can either promote PKA-mediated phosphorylation of Cx36 at regulatory sites, leading to an increase in coupling, or to decoupling via PKA-activation of protein phosphatase 2A and subsequent de-phosphorylation of Cx36109, thus providing a mechanism for bi-directional regulation of synaptic strength. Dopamine can also act via activation of D2 receptors, which leads to a reduction of cAMP and PKA activation, and increases coupling between rods and cones in vertebrate retina110. Other neuromodulatory systems that regulate the strength of electrical synapses include noradrenaline111, serotonin112,113, histamine114 and nitric oxide115,116.
Figure 4. Modalities of interactions between electrical and chemical synapses in the adult nervous system.
a, Neurotransmitter modulators released by nearby synaptic terminals (orange) regulate the synaptic strength of chemical and electrical synapses via activation of G-protein coupled metabotropic receptors. Regulation at chemical synapses could occur pre- or postsynaptically. b, Electrical and chemical synapses co-exist at mixed synapses. Glutamatergic synapses regulate the strength of electrical synapses via a postsynaptic mechanism that includes the activation of NMDA receptors (NMDAR) and CaMKII. c, Regulation of electrical synapses by glutamatergic transmission could also be heterosynaptic. Nearby glutamatergic synapses can regulate electrical transmission via NMDAR or mGLUR activation. d, Another mechanism of interaction at goldfish mixed synapses results when synaptic activity leads to mGluR activation, which in turn triggers endocannabinoid (eCB) release from the postsynaptic cell, and activates cannabinoid type-1 receptors (CB1Rs) on nearby dopaminergic fibers. CB1R activation leads to dopamine release that, by activating postsynaptic dopamine D1/5 receptors (D1/5R) and increasing PKA activity, is responsible for simultaneous enhancement of electrical and glutamatergic synaptic transmission.
Regulation of electrical coupling by neuromodulators can have profound functional consequences. Chemical and electrical synapses co-exist at various circuits raising the question of how these two networks interact to generate function117. In the rabbit retina rod and cone inputs were shown to converge on cone bipolar cells102. In the case of the cones, the connection is direct via ON bipolar cells, whereas for the rods, the connection is indirect. This indirect connection specifically relies on electrical synapses between AII type amacrine cells themselves and ON bipolar cells118. Both homologous and heterologous synapses are regulated by dopamine (AII to AII gap junctions) and nitric oxide (AII to On bipolar gap junctions), which efficiently reconfigure retinal circuits by switching between direct and indirect pathways118,119.
Remarkably, it has been shown that fast, amino acid-mediated, chemical transmission also interacts with electrical synapses. “Mixed” chemical and electrical synapses found at “Large Myelinated Club endings”, or simply “Club endings”120 -- terminations of primary auditory afferents on the teleost Mauthner cells -- provide a unique opportunity to study interactions between these two modalities of synaptic transmission (Fig. 4b)120,121. Activation of these synapses with high-frequency bursts (500 Hz) of activity leads to long lasting potentiation of both the electrical and glutamatergic components of the mixed synaptic potential evoked by the activation of these terminals122,123. Thus, both forms of transmission at these contacts exhibit activity-dependent plasticity. Strikingly, potentiation of both electrical and chemical transmission were blocked by NMDAR antagonists122,123, suggesting that potentiation of electrical transmission is initiated by the activity of the coexisting glutamatergic synapses. The potentiations also required of an increase in postsynaptic Ca++ and the activation of CaMKII124 (Fig. 4b). CaMKII activation was shown to lead to an increase in gap junction conductance not only at goldfish Club endings, but also in Cx36-containing gap junctions and cell expression systems125. Consistent with these findings, CaMKII was found to phosphorylate Cx36 at retinal electrical synapses at sites that enhance coupling between these cells126. CaMKII is an abundant component of the PSD at chemical synapses and has been implicated in mechanisms of activity-dependent plasticity at chemical synapses50,127. In similarity to its interaction with the NR2B subunit of the NMDAR at PSDs, CaMKII interacts with Cx36 by binding to its cytoplasmic domains51,127. There are multiple phosphorylation sites in Cx36 and its fish homologs Cx35 and Cx34.7 for the alpha subunit of CaMKII51,52. Residues S315/ S298/ S300 in Cx36, Cx35 and Cx34.7, respectively, constitute exclusive phosphorylation sites for CaMKII that are not shared with other kinases51,52. In contrast, residues S110 and S293 in Cx36, S110 and S276 in Cx35, and S110 and S277 in Cx34.7, are shared with PKA51,52.
Mixed synapses provided unambiguous evidence that glutamatergic and electrical synapses interact. Because interactions occur postsynaptically (Fig. 4b), it is possible that that similar interactions could also take place heterosynaptically, between neighboring glutamatergic and electrical synapses (Fig. 4c). Supporting this possibility, ultrastructural evidence shows the proximity of NR1-containing PSDs to gap junctions in the rat inferior olive and retina, at distances that comparable to those observed in goldfish Club endings128,129. Indeed NMDAR/CaMKII-dependent modulation of gap junction coupling was proposed to take place in the inferior olive128,130 and shown more recently to occur in the retina126. That is, presynaptic activity of glutamatergic ON bipolar cells increases phosphorylation of Cx36 in amacrine AII cells126. Glutamatergic synapses were also shown to induce an increase in dye coupling between hypothalamic neurons131. Moreover, glutamatergic transmission was shown to promote activity-dependent long-term depression of electrical coupling, indicating that interactions between glutamatergic and electrical synapses are diverse and widespread. High-frequency activation of cortico-thalamic inputs triggered a long-term depression of electrical transmission between thalamic relay neurons, which required the activation of metabotropic glutamate receptors132.
Interestingly, the interactions between chemical and electrical synapses were Club ending-specific and did not spread to neighboring Club endings133, suggesting that the mechanisms of PSD-initiated interactions with gap junctions are short-ranged and take place within less than 5 microns, the usual distance from two neighboring Club endings in the smooth Mauthner cell dendrite. Given that plastic changes triggered by glutamate are terminal specific and Club ending afferents can be differentially activated by sounds, the gap junctions at these contacts co-exist at different degrees of conductance on the Mauthner cell dendrite133 These findings suggest that a single cell can be differentially coupled to its neighbours based on the activity of chemical synapses located in the proximity of each gap junction. Inferior olivary cells, which connect to up to 40 other inferior olivary cells, have been shown to be differentially coupled with its partners. Such heterogeinity in coupling strength has also been reported in the, rabbit retina109, and rat cerebellar cortex134. In summary, by regulating nearby gap junctions, PSDs at glutamatergic synapses provide a mechanism for fine tuning electrical coupling within networks of electrically coupled neurons. This form of regulation can reconfigure networks and create functional compartments of neurons with propensity to cooperate, by determining the probability with which neurons interact with each other.
Heterosynaptic interactions between electrical and chemical synapses can be very complex and both involve intricate interactions between glutamatergic synapses and neurotransmitter modulators. High frequency stimulation of Club endings potentiates both electrical and chemical transmission. This effect is dependent on the activation of mGLUR-1 receptors135 and the formation of endocannabinoids, which by acting on cannabinoid type-1 receptors (CB1R) lead to the release of dopamine from varicosities located in the close vicinity of the Club endings and Mauthner cell dendrites135. The release of dopamine from nearby varicosities in turn leads to the potentiation of the electrical (and glutamatergic) synaptic response via a cAMP/PKA-mediated postsynaptic mechanism135 (Fig. 4d). This mechanism of potentiation clearly illustrates the complexity and possibilities of interactions between chemical and electrical synapses. It also demonstrates that the local availability of dopamine is not exclusively under the control of dopaminergic neurons, but that it is also determined by local synaptic activity (both electrical and chemical).
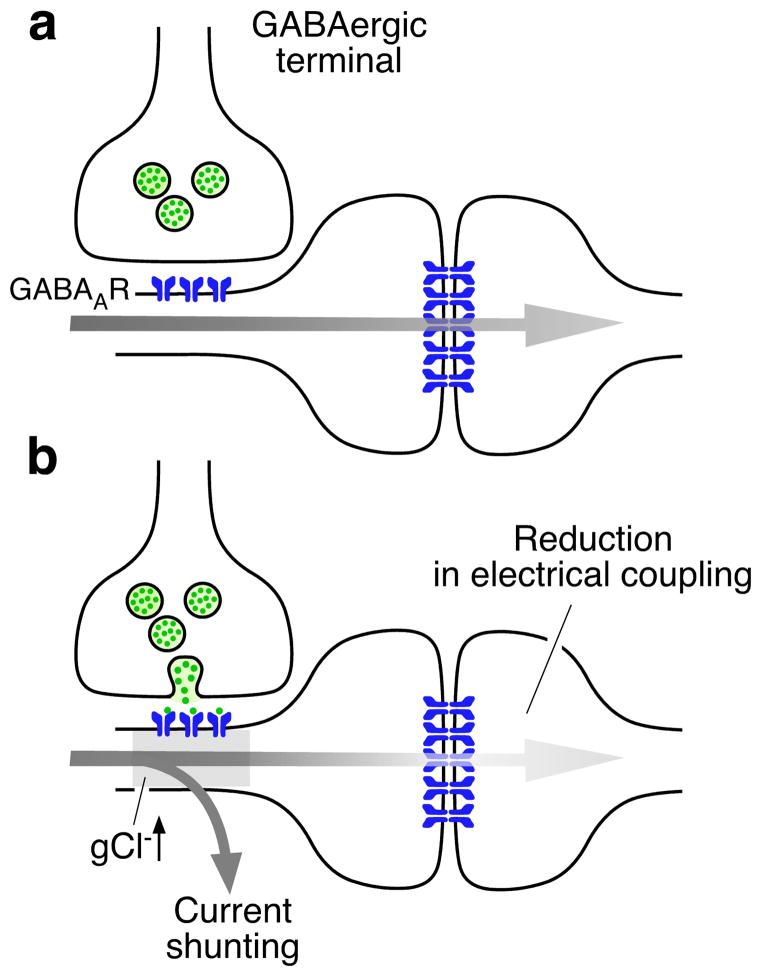
Finally, the “shunting” effect of inhibitory synapses provides another example of interactions between chemical and electrical synapses136–138. Chemical inhibitory synaptic conductance often short-circuits the currents that are generated at adjacent excitatory synapses by locally increasing membrane conductance136,138. Depending on the anatomical arrangement, inhibitory conductances can dramatically reduce coupling if they are in the proximity of electrical synapses by “shunting” electrical currents, before they have had the chance to spread to a nearby neuron through the gap junctions (Fig. 5). [See Ref. 15 for review]16 This form of interaction was shown to occur within the inferior olive glomerulus136,137 and between expansion motorneurons of the mollusk Navanax138, where quick and transient decoupling sculpts clusters of functionally related neurons involved in phasic behaviors. Further emphasizing the functional significance of these fast interactions, reciprocal electrical and inhibitory synapses organize networks of mammalian GABAergic interneurons139. Modeling one of these interneuron networks in the cerebellum suggested that combining fast inhibitory synapses and electrical coupling can promote synchronized gamma oscillations139, which have been associated with cognitive processing and are affected in some pathological conditions140.
Figure 5. Interactions between electrical synapses and inhibitory chemical synapses.
a, Inhibitory GABAergic synapses are often located in the vicinity of gap-junctions between dendro-dendritic processes (spines) of two neurons. b, By locally increasing membrane conductance, inhibitory synaptic chloride conductances (gCl−) produced by activation of GABARs briefly shunt excitatory currents to temporarily reduce effective electrical coupling between two coupled neurons.
Are interactions between electrical and chemical synapses involved in brain dysfunction?
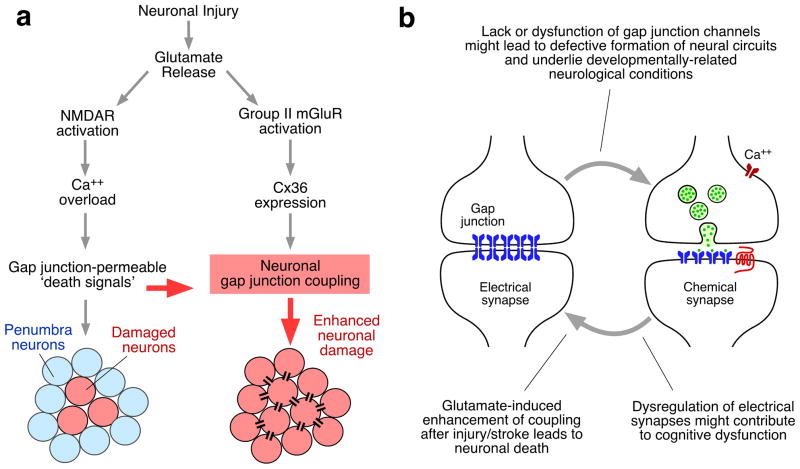
The strong developmental interrelationship between electrical and chemical synapses suggests that its disruption might underlie neurological conditions that are acquired during development. Lack or dysfunction of electrical transmission could lead, as shown in neocortex97 and the Cx36 KO mice96, to subtle deficits in the formation of mature neural circuits that may cause or contribute to neurological or behavioral conditions. Interestingly, the Cx36 gene has been associated with juvenile myoclonic epilepsy, a generalized form of epilepsy with onset in early adolescence141,142 but the exact mechanistic linkage remains unknown. It has been suggested that some single nucleotide polymorphisms may influence Cx36 gene expression and contribute to the pathogenesis of this disease by affecting the normal formation of neural circuits141. In addition, dysfunction of glutamatergic transmission, which via NMDAR activation triggers the developmental decline in gap junctional communication99, could result in abnormal levels of electrical coupling in some brain regions that might lead to increased neuronal synchrony and seizures143.
As previously mentioned, Cx36-mediated electrical synapses are a feature of inhibitory interneurons in the adult mammalian brain, including neo-cortex, thalamus and hippocampus6–8,132. Inhibitory interneurons are crucial for various brain processes, as they contribute to the generation of gamma-frequency oscillatory activity (30–80 Hz), which has been associated with cognitive processing140,144. High-frequency network oscillatory synchronizations appear to be crucial in defining the conscious state145, and for “associative binding” for learning and memory. Strikingly, these cortical gamma oscillations are impaired in Cx36 knockout mice146 suggesting a role for electrical synapses in generating this rhythm. Alteration of oscillatory activity has been proposed to contribute to the pathophysiology of schizophrenia144,147, Parkinson’s disease148 and autism spectral disorders149. Moreover, dysfunction of glutamatergic150–152 and dopaminergic153,154 transmissions, are involved in these conditions, suggesting that dysregulation of electrical coupling could contribute to the underlying pathophysiological processes. The investigation of these possibilities could lead to the identification of novel therapeutic opportunities for treating these conditions.
Recent data suggest that the interactions between chemical and electrical synapses that are observed during development have an important functional role after brain insult. Strikingly, networks of electrically coupled neurons identified during development can reappear after brain injury. Adult cat spinal motorneurons become re-coupled by gap junctions after peripheral nerve injury155, reestablishing the transient gap junction network observed during development. Gap junction coupling was proposed to play a beneficial functional role by helping to maintain the viability of axotomized motorneurons until synaptic connections with their muscle fiber targets are reestablished155. Although it is unclear if this axotomy-induced spinal network disappears after nerve regeneration, glutamatergic transmission was shown to increase expression of Cx36 and gap junction communication in in-vivo and in-vitro models of ischemic stroke and traumatic brain injury101. The increase in Cx36 expression occurs about 2 hours after injury and is likely to be triggered by the massive release of glutamate from injured neurons101. As observed during development100, activation of group II mGluRs was also shown to mediate this increase in gap junction communication156.
These interactions between glutamatergic and electrical transmission are thought to control death and survival mechanisms in injured neurons156. A wealth of data indicate that activation of NMDA receptors mediate the glutamate toxicity that occurs in the injured brain157. Interestingly, electrical synapses seem to be involved in this phenomenon. The death of forebrain neurons evoked by intraperitoneal injection of NMDA was greatly attenuated by gap junction blockers and in Cx36 KO mice158,159, suggesting that gap junctional coupling could have a deleterious effect for neuronal survival. It has been suggested that neurodegenerative signals could be passed to other and undamaged neurons (neurons adjacent to the lesion or “penumbra” area) as a result of the increase in neuronal coupling triggered by the activation of group II mGluRs160 (Fig. 6a).
Figure 6. Interactions between chemical and electrical synapses and pathological processes.
a, Chemical and electrical synapses interact after injury. Release of glutamate from injured neurons causes neuronal damage via NMDAR activation and Ca++ overload at the site of the lesion. Simultaneous enhancement of coupling via mGluR-dependent increased expression of Cx36 extends neuronal damage by facilitating the passage of ‘death signals’ to neurons adjacent to the lesion or “penumbra” area (from Belousov and Fontes et al., 2013). b, Summary of potential interactions of chemical and electrical synapses during pathological processes. The lack, or dysfunction, of gap junction channels at early developmental stages might lead to defective formation of critical neural circuits formed by chemical synapses, underlying developmentally-related neurological conditions. During adulthood, release of glutamate from compromised neurons after injury or stroke enhances coupling which leads to neuronal death in uncompromised areas adjacent to the lesion. Dysregulation of electrical synapses strength by neurotransmitters might contribute to cognitive disorders.
Conclusions
Electrical synapses have proven to be more widespread in the mammalian brain than originally anticipated. Thus, networks of electrically and chemically coupled neurons are not restricted to invertebrates but seem to be a feature of all nervous systems. Such realization emphasizes the need to further explore the functional properties of electrical synapses (of which we know significantly less) as well, based on the evidence reviewed here, their interactions with chemical synapses.
Mixed synapses provide unambiguous evidence that rather than unconnected lines of communication electrical and chemical synapses cooperate and extensively interact, and that the speed and reliability of electrical transmission can be combined with the ability to induce plastic changes that is characteristic of chemical transmission. Mixed synapses are widespread in cold-blooded vertebrates120 but they seem to be less numerous in mammals, perhaps because of the much-improved speed of transmission observed at mammalian chemical synapses161. However, there is growing evidence of their presence in the mammalian nervous system including the spinal cord162, a subset of hippocampal mossy fibers163,164, and, interestingly, between dendrites of hippocampal inhibitory interneurons165.
Interactions between electrical and chemical synapses are complex and diverse, and occur at all stages of brain development. While during development interactions between electrical synapses are critical for the formation on neural circuits, interactions in the adult brain result in dynamic reconfiguration of hardwired networks. These interactions are likely to have important pathological implications (Fig. 6b). Recapitulation of some developmental interactions between chemical and electrical synapses after brain injury indicates that, rather than exceptional, these interactions constitute basic and necessary mechanisms of communication in the nervous system. Furthermore, the interactions between dopaminergic, glutamatergic and electrical synapses that have been observed in the adult brain highlight their importance for reconfiguring neural circuits and their dysregulation could contribute to cognitive impairment. Future studies are likely to confirm the intimate relationship between these two modalities of synaptic transmission and shed further light on their contribution to disease states.
At-a-glance Summary.
There are two main modalities of synaptic transmission: chemical and electrical. Although chemical synapses are perceived as structurally more complex and functionally dynamic, emerging evidence indicates that electrical synapses might be similarly complex, functionally diverse and highly modifiable.
Far from functioning independently and serving unrelated functions, these two modalities of synaptic transmission closely interact. Rather than conceiving synaptic transmission as either chemical or electrical, this article emphasizes the notion that synaptic transmission is both chemical and electrical and that interactions between these two forms of interneuronal communication are required for normal brain development and function.
The development of neural circuits in disparate nervous systems (both vertebrate and invertebrate) seems to critically rely on interactions between chemical and electrical synapses, which reciprocally and dynamically regulate the emergence of these two forms of transmission.
While during development interactions between electrical synapses are critical for the formation on neural circuits, interactions in the adult brain result in dynamic reconfiguration of hardwired networks. The strength of electrical synapses is regulated by neurotransmitters modulators such as dopamine and by glutamatergic synapses in an activity-dependent fashion.
Interactions between electrical and chemical synapses are also likely to have important pathological implications. Recapitulation of developmental interactions between chemical and electrical synapses was observed after brain injury and dysregulation of electrical synapses by neurotransmitters could contribute to cognitive impairment.
Acknowledgments
This research was supported by National Institutes of Health grants NIH DC03186, DC011099, R21NS055726, R21NS085772 and NS0552827 to A.E.P.
Glossary terms
- Lateral excitation
The term lateral inhibition refers to the ability of an excited neuron to inhibit or reduce the activity of its neighbors. Lateral inhibition following activation of a sensory afferent is thought to increase spatiotemporal perceptual discrimination. Lateral excitation, a less appreciated property of sensory and cortical networks, refers to the ability of an excited neuron (or sensory afferent) to excite its neighbors. While reducing discrimination, lateral excitation greatly enhances input sensitivity
- Escape networks
Neural networks found in invertebrate and vertebrate nervous systems that seem optimized to mediate fast escape behaviors. They usually contain a small number of cells and include sensory and motor neurons
- Mauthner cell
The Mauthner cells are two large reticulospinal neurons found in teleost fishes that mediate (amongst other functions) tail-flip sensory evoked escape responses
- Postsynaptic density (PSD)
Originally named after its identification by electron microscopy, the term refers to a macromolecular complex that supports postsynaptic function at chemical synapses. Better characterized at glutamatergic synapses, the PSD is formed by a large number of proteins that include neurotransmitters receptors, scaffolding proteins and regulatory signaling molecules
- Electroretinogram
Extracellularly recorded electrical response that reflects the activation of various cells in the retina (including photoreceptors, inner retinal cells, and the output ganglion cells) in response to visual stimulation
- ON bipolar cells
Retinal cell that functionally link photoreceptors (cones and rods) with ganglion cells. ON bipolar cells are excited by the release of glutamate from photoreceptors while OFF bipolar cells are instead inhibited
- All type amacrine cells
Amacrine cells represent a class of retinal interneurons that represent the main input to ganglion cells, the output neuron of the retina. Amacrine cells also regulate bipolar cells, which represent the other source of input to ganglion cells. The AII is a type of amacrine cell that relays rod-driven information through the ON-center cone bipolar axons to ON-center ganglion cells via electrical synapses
- Associative binding
The term refers to tasks of episodic memory that require the associative binding of distinct components into a compound episode. They commonly include the binding of two items or a single item with a specific context
Biography
Alberto Pereda is a Professor of Neuroscience at the Albert Einstein College of Medicine (Bronx, New York). His postdoctoral training focused on investigating mechanisms of postural control during sleep (with Michael Chase and Francisco Morales at UCLA) and the plastic properties of auditory synapses on the goldfish Mauthner cells (with Donald Faber at the University at Buffalo). His current research efforts are centered on investigating the properties and mechanisms of plasticity of gap junction-mediated electrical synapses in the vertebrate brain.
References
- 1.The Synapse by Bernardo Sabatini Morgan Sheng | 9781936113026 | Hardcover | Barnes & Noble.
- 2.Bennett MVL, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 3.Zoli M, et al. The emergence of the volume transmission concept. Brain Res Brain Res Rev. 1998;26:136–47. doi: 10.1016/s0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]
- 4.Faber DS, Korn H. Electrical field effects: their relevance in central neural networks. Physiol Rev. 1989;69:821–63. doi: 10.1152/physrev.1989.69.3.821. [DOI] [PubMed] [Google Scholar]
- 5.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 6.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–5. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 7.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–9. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 8.Galarreta M, Hestrin S. Electrical synapses between Gaba-Releasing interneurons. Nat Rev Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- 9.Bennett MV. Electrical synapses, a personal perspective (or history) Brain Res Brain Res Rev. 2000;32:16–28. doi: 10.1016/s0165-0173(99)00065-x. [DOI] [PubMed] [Google Scholar]
- 10.Bandara HMHN, Lam OLT, Jin LJ, Samaranayake L. Microbial chemical signaling: a current perspective. Crit Rev Microbiol. 2012;38:217–49. doi: 10.3109/1040841X.2011.652065. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Nair SK. Quorum sensing: how bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. 2012;21:1403–17. doi: 10.1002/pro.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dustin ML. Signaling at neuro/immune synapses. J Clin Invest. 2012;122:1149–55. doi: 10.1172/JCI58705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–9. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Goodenough Da, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Pereda AE, et al. Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity. Biochim Biophys Acta. 2013;1828:134–46. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudò G. Expression of Cx36 in mammalian neurons. Brain Res Brain Res Rev. 2000;32:72–85. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 18.Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–45. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Kandarian B, et al. The medicinal leech genome encodes 21 innexin genes: different combinations are expressed by identified central neurons. Dev Genes Evol. 2012;222:29–44. doi: 10.1007/s00427-011-0387-z. [DOI] [PubMed] [Google Scholar]
- 20.Liu P, et al. Six Innexins Contribute to Electrical Coupling of C. elegans Body-Wall Muscle. PLoS One. 2013;8:e76877. doi: 10.1371/journal.pone.0076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Getting PA. Modification of neuron properties by electrotonic synapses. I Input resistance, time constant, and integration. J Neurophysiol. 1974;37:846–57. doi: 10.1152/jn.1974.37.5.846. [DOI] [PubMed] [Google Scholar]
- 22.Getting PA, Willows AO. Modification of neuron properties by electrotonic synapses. II Burst formation by electrotonic synapses. J Neurophysiol. 1974;37:858–68. doi: 10.1152/jn.1974.37.5.858. [DOI] [PubMed] [Google Scholar]
- 23.Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–9. doi: 10.1126/science.1061395. The authors proposed that electrical synapses operate as coincidence detectors in networks of electrically-coupled neurons. [DOI] [PubMed] [Google Scholar]
- 24.Veruki ML, Hartveit E. AII (Rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002;33:935–46. doi: 10.1016/s0896-6273(02)00609-8. [DOI] [PubMed] [Google Scholar]
- 25.Curti S, Hoge G, Nagy JI, Pereda AE. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–59. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVries SH, Qi X, Smith R, Makous W, Sterling P. Electrical coupling between mammalian cones. Curr Biol. 2002;12:1900–7. doi: 10.1016/s0960-9822(02)01261-7. [DOI] [PubMed] [Google Scholar]
- 27.Pereda AE, Bell TD, Faber DS. Retrograde synaptic communication via gap junctions coupling auditory afferents to the Mauthner cell. J Neurosci. 1995;15:5943–55. doi: 10.1523/JNEUROSCI.15-09-05943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curti S, Pereda AE. Voltage-dependent enhancement of electrical coupling by a subthreshold sodium current. J Neurosci. 2004;24:3999–4010. doi: 10.1523/JNEUROSCI.0077-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herberholz J, Antonsen BL, Edwards DH. A lateral excitatory network in the escape circuit of crayfish. J Neurosci. 2002;22:9078–85. doi: 10.1523/JNEUROSCI.22-20-09078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vervaeke K, Lorincz A, Nusser Z, Silver RA. Gap junctions compensate for sublinear dendritic integration in an inhibitory network. Science. 2012;335:1624–8. doi: 10.1126/science.1215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phelan P, et al. Mutations in shaking-B prevent electrical synapse formation in the Drosophila giant fiber system. J Neurosci. 1996;16:1101–13. doi: 10.1523/JNEUROSCI.16-03-01101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22:153–61. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- 33.Faber DS, Pereda AE. Encycl Fish Physiol From Genome to Environ. Vol. 1. Elsevier Inc; 2011. Physiology of the Mauthner Cell3: Function; pp. 73–79. [Google Scholar]
- 34.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–43. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laird DW. The life cycle of a connexin: gap junction formation, removal, and degradation. J Bioenerg Biomembr. 1996;28:311–8. doi: 10.1007/BF02110107. [DOI] [PubMed] [Google Scholar]
- 36.Gumpert AM, Varco JS, Baker SM, Piehl M, Falk MM. Double-membrane gap junction internalization requires the clathrin-mediated endocytic machinery. FEBS Lett. 2008;582:2887–92. doi: 10.1016/j.febslet.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piehl M, et al. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell. 2007;18:337–47. doi: 10.1091/mbc.E06-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauf U, et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–51. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaietta G, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 40.Flores CE, et al. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc Natl Acad Sci U S A. 2012;109:E573–82. doi: 10.1073/pnas.1121557109. This paper represents the first evidence of trafficking of gap junction channels in a native electrical synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereda AE, Faber DS. Encycl Fish Physiol From Genome to Environ. Vol. 1. Elsevier Inc; 2011. Physiology of the Mauthner Cell3: Discovery and Properties; pp. 66–72. [Google Scholar]
- 42.Hervé JC, Derangeon M, Bahbouhi B, Mesnil M, Sarrouilhe D. The connexin turnover, an important modulating factor of the level of cell-to-cell junctional communication: comparison with other integral membrane proteins. J Membr Biol. 2007;217:21–33. doi: 10.1007/s00232-007-9054-8. [DOI] [PubMed] [Google Scholar]
- 43.Lüscher C, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–58. doi: 10.1016/s0896-6273(00)81119-8. One of the first descriptions of the existence of trafficking and cycling of receptors at glutamatergic synapses and its potenttial role in synaptic plasticity. [DOI] [PubMed] [Google Scholar]
- 44.Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–7. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- 45.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–24. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 408:936–43. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 47.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–25. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 48.Dong H, et al. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–84. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 49.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–24. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–8. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- 51.Alev C, et al. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:20964–9. doi: 10.1073/pnas.0805408105. The paper provides evidence for the existence of direct protein-protein interactions between gap junction forming proteins and CaMKII, a regulatory kinase also shown to regulate chemical synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores CE, et al. Variability of distribution of Ca(2+)/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: colocalization and association with connexin 35. J Neurosci. 2010;30:9488–99. doi: 10.1523/JNEUROSCI.4466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sotelo C, Korn H. Morphological correlates of electrical and other interactions through low-resistance pathways between neurons of the vertebrate central nervous system. Int Rev Cytol. 1978;55:67–107. doi: 10.1016/s0074-7696(08)61887-2. [DOI] [PubMed] [Google Scholar]
- 54.Lynn BD, Li X, Nagy JI. Under construction: building the macromolecular superstructure and signaling components of an electrical synapse. J Membr Biol. 2012;245:303–17. doi: 10.1007/s00232-012-9451-5. The authors review the proteins associated to connexin-formed gap junction channels at electrical synapses, which have been so far identified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Lynn BD, Nagy JI. The effector and scaffolding proteins AF6 and MUPP1 interact with connexin36 and localize at gap junctions that form electrical synapses in rodent brain. Eur J Neurosci. 2012;35:166–81. doi: 10.1111/j.1460-9568.2011.07947.x. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Lu S, Nagy JI. Direct association of connexin36 with zonula occludens-2 and zonula occludens-3. Neurochem Int. 54:393–402. doi: 10.1016/j.neuint.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Olson C, Lu S, Nagy JI. Association of connexin36 with zonula occludens-1 in HeLa cells, betaTC-3 cells, pancreas, and adrenal gland. Histochem Cell Biol. 2004;122:485–98. doi: 10.1007/s00418-004-0718-5. [DOI] [PubMed] [Google Scholar]
- 58.Li X, et al. Neuronal connexin36 association with zonula occludens-1 protein (ZO-1) in mouse brain and interaction with the first PDZ domain of ZO-1. Eur J Neurosci. 2004;19:2132–46. doi: 10.1111/j.l460-9568.2004.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flores CE, Li X, Bennett MVL, Nagy JI, Pereda AE. Interaction between connexin35 and zonula occludens-1 and its potential role in the regulation of electrical synapses. Proc Natl Acad Sci U S A. 2008;105:12545–50. doi: 10.1073/pnas.0804793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hervé JC, Bourmeyster N, Sarrouilhe D. Diversity in protein-protein interactions of connexins: emerging roles. Biochim Biophys Acta. 2004;1662:22–41. doi: 10.1016/j.bbamem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Helbig I, et al. In vivo evidence for the involvement of the carboxy terminal domain in assembling connexin 36 at the electrical synapse. Mol Cell Neurosci. 2010;45:47–58. doi: 10.1016/j.mcn.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen B, Liu Q, Ge Q, Xie J, Wang ZW. UNC-1 regulates gap junctions important to locomotion in C. elegans. Curr Biol. 2007;17:1334–9. doi: 10.1016/j.cub.2007.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norman KR, Maricq AV. Innexin function: minding the gap junction. Curr Biol. 2007;17:R812–4. doi: 10.1016/j.cub.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 65.Rash JE, et al. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron. 2013;79:957–69. doi: 10.1016/j.neuron.2013.06.037. The paper provides evidence suggesting that gap junction hemiplaques at electrical synapses might not necessarily be the mirror image of each other. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phelan P, et al. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr Biol. 2008;18:1955–60. doi: 10.1016/j.cub.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrio LC, et al. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991;88:8410–4. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello T. A Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol. 1999;114:339–64. doi: 10.1085/jgp.114.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–51. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 70.Volff JN. Genome evolution and biodiversity in teleost fish. Heredity (Edinb) 2005;94:280–94. doi: 10.1038/sj.hdy.6800635. [DOI] [PubMed] [Google Scholar]
- 71.Kandler K, Katz LC. Neuronal coupling and uncoupling in the developing nervous system. Curr Opin Neurobiol. 1995;5:98–105. doi: 10.1016/0959-4388(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 72.Montoro RJ, Yuste R. Gap junctions in developing neocortex: a review. Brain Res Brain Res Rev. 2004;47:216–226. doi: 10.1016/j.brainresrev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. The paper describes the existence of extensive gap junction coupling and its developmental regulation in the vertebrate brain. [DOI] [PubMed] [Google Scholar]
- 74.Peinado A, Yuste R, Katz LC. Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex. 1993;3:488–498. doi: 10.1093/cercor/3.5.488. [DOI] [PubMed] [Google Scholar]
- 75.Penn AA, Wong RO, Shatz CJ. Neuronal coupling in the developing mammalian retina. J Neurosci. 1994;14:3805–3815. doi: 10.1523/JNEUROSCI.14-06-03805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bittman K, Owens DF, Kriegstein AR, LoTurco JJ. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science (80- ) 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- 78.Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- 79.Marin-Burgin A, Eisenhart FJ, Baca SM, Kristan WB, French Ka. Sequential development of electrical and chemical synaptic connections generates a specific behavioral circuit in the leech. J Neurosci. 2005;25:2478–89. doi: 10.1523/JNEUROSCI.4787-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marin-Burgin A, Eisenhart FJ, Kristan WB, French KA. Embryonic electrical connections appear to pre-figure a behavioral circuit in the leech CNS. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:123–33. doi: 10.1007/s00359-005-0055-8. [DOI] [PubMed] [Google Scholar]
- 81.Wolszon L. Cell-cell interactions define the innervation patterns of central leech neurons during development. J Neurobiol. 1995;27:335–52. doi: 10.1002/neu.480270307. [DOI] [PubMed] [Google Scholar]
- 82.Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell. 2007;129:787–99. doi: 10.1016/j.cell.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 83.Baker MW, Yazdani N, Macagno ER. Gap junction-dependent homolog avoidance in the developing CNS. J Neurosci. 2013;33:16673–83. doi: 10.1523/JNEUROSCI.2387-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolszon LR, Rehder V, Kater SB, Macagno ER. Calcium wave fronts that cross gap junctions may signal neuronal death during development. J Neurosci. 1994;14:3437–48. doi: 10.1523/JNEUROSCI.14-06-03437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolszon LR, Gao WQ, Passani MB, Macagno ER. Growth cone “collapse” in vivo: are inhibitory interactions mediated by gap junctions? J Neurosci. 1994;14:999–1010. doi: 10.1523/JNEUROSCI.14-03-00999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolszon LR, Passani MB, Macagno ER. Interactions during a critical period inhibit bilateral projections in embryonic neurons. J Neurosci. 1995;15:1506–15. doi: 10.1523/JNEUROSCI.15-02-01506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap Junctional Coupling and Patterns of Connexin Expression among Neonatal Rat Lumbar Spinal Motor Neurons. J Neurosci. 1999;19:10813–10828. doi: 10.1523/JNEUROSCI.19-24-10813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Personius K, Chang Q, Bittman K, Panzer J, Balice-Gordon R. Gap junctional communication among motor and other neurons shapes patterns of neural activity and synaptic connectivity during development. Cell Commun Adhes. 2001;8:329–33. doi: 10.3109/15419060109080748. [DOI] [PubMed] [Google Scholar]
- 89.Walton KD, Navarrete R. Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat lumbar spinal cord. J Physiol. 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colman H, Lichtman JW. Interactions between nerve and muscle: synapse elimination at the developing neuromuscular junction. Dev Biol. 1993;156:1–10. doi: 10.1006/dbio.1993.1054. [DOI] [PubMed] [Google Scholar]
- 91.Personius KE, Balice-Gordon RJ. Loss of correlated motor neuron activity during synaptic competition at developing neuromuscular synapses. Neuron. 2001;31:395–408. doi: 10.1016/s0896-6273(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 92.Personius KE, Chang Q, Mentis GZ, O’Donovan MJ, Balice-Gordon RJ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci U S A. 2007;104:11808–13. doi: 10.1073/pnas.0703357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szabo TM, Faber DS, Zoran MJ. Transient electrical coupling delays the onset of chemical neurotransmission at developing synapses. J Neurosci. 2004;24:112–120. doi: 10.1523/JNEUROSCI.4336-03.2004. Using a reduced invertebrate model, the authors unambiguosly demostrate the interelationship between the formation of electrical and chemical synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Todd KL, Jr, WBK, French KA. Gap Junction Expression Is Required for Normal Chemical Synapse Formation. 2010;30:15277–15285. doi: 10.1523/JNEUROSCI.2331-10.2010. It elegantly demostrates the initial requirement of electrical synapses for the formation of chemical synapses in an in-vivo system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mentis GZ, Díaz E, Moran LB, Navarrete R. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J Physiol. 2002;544:757–64. doi: 10.1113/jphysiol.2002.028159. The paper provides evidence for the existence of an inverse relationship between the presence of electrical synapses and chemical synapses in the mammalian brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maher BJ, McGinley MJ, Westbrook GL. Experience-dependent maturation of the glomerular microcircuit. Proc Natl Acad Sci U S A. 2009;106:16865–70. doi: 10.1073/pnas.0808946106. The paper demonstrates the existence of deficits in the formation of circuits formed by chemical synapses in mice lacking the gap junction protein Cx36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu YC, et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature. 2012;486:113–7. doi: 10.1038/nature10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Curtin KD, Zhang Z, Wyman RJ. Gap Junction Proteins Expressed during Development Are Required for Adult Neural Function in the Drosophila Optic Lamina. J Neurosci. 2002;22:7088–7096. doi: 10.1523/JNEUROSCI.22-16-07088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8:1720–6. doi: 10.1038/nn1588. The paper describes at the mechanistic level the how the emergence of glutamatergic transmission leads to a massive reduction of gap junction coupling in the developing brain. [DOI] [PubMed] [Google Scholar]
- 100.Park WM, et al. Interplay of chemical neurotransmitters regulates developmental increase in electrical synapses. J Neurosci. 2011;31:5909–20. doi: 10.1523/JNEUROSCI.6787-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Belousov AB, Fontes JD. Neuronal gap junctions: making and breaking connections during development and injury. Trends Neurosci. 2013;36:227–36. doi: 10.1016/j.tins.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci U S A. 1992;89:12088–92. doi: 10.1073/pnas.89.24.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pereda AE, Nairn AC, Wolszon LR, Faber DS. Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell. J Neurosci. 1994;14:3704–12. doi: 10.1523/JNEUROSCI.14-06-03704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3′:5′-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984;4:2477–88. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985;82:3025–9. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Urschel S, et al. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–71. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- 108.Kothmann WW, Li X, Burr GS, O’Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–11. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci. 2008;28:1804–15. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rörig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. Eur J Neurosci. 1996;8:1685–1695. doi: 10.1111/j.1460-9568.1996.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 113.Johnson BR, Peck JH, Harris-Warrick RM. Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol A. 1993;172:715–32. doi: 10.1007/BF00195397. [DOI] [PubMed] [Google Scholar]
- 114.Hatton GI, Yang QZ. Synaptically released histamine increases dye coupling among vasopressinergic neurons of the supraoptic nucleus: mediation by H1 receptors and cyclic nucleotides. J Neurosci. 1996;16:123–9. doi: 10.1523/JNEUROSCI.16-01-00123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Donnell P, Grace AA. Cortical afferents modulate striatal gap junction permeability via nitric oxide. Neuroscience. 1997;76:1–5. doi: 10.1016/s0306-4522(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 116.Rörig B, Sutor B. Nitric oxide-stimulated increase in intracellular cGMP modulates gap junction coupling in rat neocortex. Neuroreport. 1996;7:569–72. doi: 10.1097/00001756-199601310-00046. [DOI] [PubMed] [Google Scholar]
- 117.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–65. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 118.Xia X-B, Mills SL. Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci. 21:791–805. doi: 10.1017/S0952523804045122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–7. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- 120.Pereda AE, Rash JE, Nagy JI, Bennett MVL. Dynamics of electrical transmission at club endings on the Mauthner cells. Brain Res Brain Res Rev. 2004;47:227–44. doi: 10.1016/j.brainresrev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 121.Pereda a, et al. Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells. J Neurosci. 2003;23:7489–503. doi: 10.1523/JNEUROSCI.23-20-07489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang XD, Korn H, Faber DS. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990;348:542–5. doi: 10.1038/348542a0. This study provided the first evidence for the existence of activity-dependent potentiation in electrical synapses. [DOI] [PubMed] [Google Scholar]
- 123.Pereda AE, Faber DS. Activity-dependent short-term enhancement of intercellular coupling. J Neurosci. 1996;16:983–92. doi: 10.1523/JNEUROSCI.16-03-00983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pereda aE, et al. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gap-junctional conductance and glutamatergic transmission. Proc Natl Acad Sci U S A. 1998;95:13272–7. doi: 10.1073/pnas.95.22.13272. The paper provides the first evidence for the role of CaMKII in regulating electrical transmission, a key regulatory kinase also involved in activity-dependent regulation of glutamatergic synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Del Corsso C, Iglesias R, Zoidl G, Dermietzel R, Spray DC. Calmodulin dependent protein kinase increases conductance at gap junctions formed by the neuronal gap junction protein connexin36. Brain Res. 2012;1487:69–77. doi: 10.1016/j.brainres.2012.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kothmann WW, et al. Nonsynaptic NMDA receptors mediate activity-dependent plasticity of gap junctional coupling in the AII amacrine cell network. J Neurosci. 2012;32:6747–59. doi: 10.1523/JNEUROSCI.5087-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoge GJ, et al. The extent and strength of electrical coupling between inferior olivary neurons is heterogeneous. J Neurophysiol. 2011;105:1089–101. doi: 10.1152/jn.00789.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rash JE, et al. High-resolution proteomic mapping in the vertebrate central nervous system: close proximity of connexin35 to NMDA glutamate receptor clusters and co-localization of connexin36 with immunoreactivity for zonula occludens protein-1 (ZO-1) J Neurocytol. 2004;33:131–51. doi: 10.1023/B:NEUR.0000029653.34094.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Urbano FJ, Leznik E, Llinás RR. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci U S A. 2007;104:12554–9. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hatton GI, Yang QZ. Activation of excitatory amino acid inputs to supraoptic neurons. I Induced increases in dye-coupling in lactating, but not virgin or male rats. Brain Res. 1990;513:264–9. doi: 10.1016/0006-8993(90)90465-n. [DOI] [PubMed] [Google Scholar]
- 132.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–13. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 133.Smith M, Pereda AE. Chemical synaptic activity modulates nearby electrical synapses. Proc Natl Acad Sci U S A. 2003;100:4849–54. doi: 10.1073/pnas.0734299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vervaeke K, et al. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron. 2010;67:435–51. doi: 10.1016/j.neuron.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cachope R, Mackie K, Triller A, O’Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–47. doi: 10.1016/j.neuron.2007.11.014. The article describes interactions between glutamatergic, dopaminergic and electrical synapses, demonstrating a complex functional interelationship between chemical and electrical trasmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–71. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 137.Best AR, Regehr WG. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron. 2009;62:555–65. doi: 10.1016/j.neuron.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spira ME, Spray DC, Bennett MV. Electrotonic coupling: effective sign reversal by inhibitory neurons. Science. 1976;194:1065–7. doi: 10.1126/science.185698. [DOI] [PubMed] [Google Scholar]
- 139.Bartos M, et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99:13222–7. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mas C, et al. Association of the connexin36 gene with juvenile myoclonic epilepsy. J Med Genet. 2004;41:e93. doi: 10.1136/jmg.2003.017954. First evidence of the relationship between the Cx36 gene and juvenile myoclonic epilepsy, suggesting the existence of developmentally related deficits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hempelmann A, Heils A, Sander T. Confirmatory evidence for an association of the connexin-36 gene with juvenile myoclonic epilepsy. Epilepsy Res. 2006;71:223–8. doi: 10.1016/j.eplepsyres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 143.Dudek FE, Snow RW, Taylor CP. Role of electrical interactions in synchronization of epileptiform bursts. Adv Neurol. 1986;44:593–617. [PubMed] [Google Scholar]
- 144.Nakazawa K, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–83. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- 146.Hormuzdi SG, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–95. doi: 10.1016/s0896-6273(01)00387-7. The study provides evidence for the contribution of electrical coupling between cortical inhibitory interneurons in the generation of gamma oscillations, which are associated to cognitive phenomena. [DOI] [PubMed] [Google Scholar]
- 147.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–44. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–64. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 149.Welsh JP, Ahn ES, Placantonakis DG. Is autism due to brain desynchronization? Int J Dev Neurosci. 23:253–63. doi: 10.1016/j.ijdevneu.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 150.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–4. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 151.Javitt DC, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:102mr2. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 154.Penzes P, Buonanno A, Passafaro M, Sala C, Sweet RA. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J Neurochem. 2013;126:165–82. doi: 10.1111/jnc.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang Q, Pereda A, Pinter MJ, Balice-Gordon RJ. Nerve injury induces gap junctional coupling among axotomized adult motor neurons. J Neurosci. 2000;20:674–84. doi: 10.1523/JNEUROSCI.20-02-00674.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Y, et al. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J Neurosci. 2012;32:713–25. doi: 10.1523/JNEUROSCI.3872-11.2012. The authors describe the relationship between glutamate and increased gap junctional coupling observed after neuronal injury and its underlying mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941–53. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 158.Belousov AB, et al. Neuronal gap junctions play a role in the secondary neuronal death following controlled cortical impact. Neurosci Lett. 2012;524:16–9. doi: 10.1016/j.neulet.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Y, et al. Neuronal gap junctions are required for NMDA receptor-mediated excitotoxicity: implications in ischemic stroke. J Neurophysiol. 2010;104:3551–6. doi: 10.1152/jn.00656.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Belousov AB. Novel model for the mechanisms of glutamate-dependent excitotoxicity: role of neuronal gap junctions. Brain Res. 2012;1487:123–30. doi: 10.1016/j.brainres.2012.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–2. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- 162.Rash JE, et al. Mixed synapses discovered and mapped throughout mammalian spinal cord. Proc Natl Acad Sci U S A. 1996;93:4235–9. doi: 10.1073/pnas.93.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hamzei-Sichani F, et al. Mixed Electrical-Chemical Synapses in Adult Rat Hippocampus are Primarily Glutamatergic and Coupled by Connexin-36. Front Neuroanat. 2012;6:13. doi: 10.3389/fnana.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vivar C, Traub RD, Gutiérrez R. Mixed electrical-chemical transmission between hippocampal mossy fibers and pyramidal cells. Eur J Neurosci. 2012;35:76–82. doi: 10.1111/j.1460-9568.2011.07930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fukuda T, Kosaka T. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J Neurosci. 2000;20:1519–28. doi: 10.1523/JNEUROSCI.20-04-01519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Valenstein E. The War of the Soups and the Sparks: The Discovery of Neurotransmitters and the Dispute Over How Nerves Communicate. Columbia University Press; 2006. p. 256. [Google Scholar]
- 167.Elliott TR. The action of adrenalin. J Physiol. 1905;32:401–67. doi: 10.1113/jphysiol.1905.sp001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Loewi O. Über humorale Übertragbarkeit der Herznervenwirkung. Pflugers Arch Gesamte Physiol Menschen Tiere. 1924;204:629–640. [Google Scholar]
- 169.Sherrington CS. The Integrative Action of the Nervous System - Primary Source Edition. Nabu Press; 2013. p. 436. [Google Scholar]
- 170.Katz B. The Release of Neural Transmitter Substances (Sherrington Lecture) Liverpool University Press; 1969. p. 70. [Google Scholar]
- 171.Furshapn EJ, Potter DD. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959;145:289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bennett MV, Aljure E, Nakajima Y, Pappas GD. Electrotonic junctions between teleost spinal neurons: electrophysiology and ultrastructure. Science. 1963;141(80):262–264. doi: 10.1126/science.141.3577.262. [DOI] [PubMed] [Google Scholar]
- 173.Robertson JD, Bodenheimer TS, Stage DE. The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J Cell Biol. 1963;19:159–99. doi: 10.1083/jcb.19.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Furshpan EJ. “Electrical Transmission” At an Excitatory Synapse in a Vertebrate Brain. Science. 1964;144:878–80. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]