Abstract
Studies have demonstrated that the induction of oxidative stress may be involved in brain tumor induction in rats by acrylonitrile. The present study examined whether acrylonitrile induces oxidative stress and DNA damage in rats and whether blood can serve as a valid surrogate for the biomonitoring of oxidative stress induced by acrylonitrile in the exposed population. Male Sprague-Dawley rats were treated with 0, 3, 30, 100, and 200 ppm acrylonitrile in drinking water for 28 days. One group of rats were also coadministered N-acetyl cysteine (NAC) (0.3% in diet) with acrylonitrile (200 ppm in drinking water) to examine whether antioxidant supplementation was protective against acrylonitrile-induced oxidative stress. Direct DNA strand breakage in white blood cells (WBC) and brain was measured using the alkaline comet assay. Oxidative DNA damage in WBC and brain was evaluated using formamidopyrimidine DNA glycosylase (fpg)-modified comet assay and with high-performance liquid chromatography-electrochemical detection. No significant increase in direct DNA strand breaks was observed in brain and WBC from acrylonitrile-treated rats. However, oxidative DNA damage (fpg comet and 8′hydroxyl-2-deoxyguanosine) in brain and WBC was increased in a dose-dependent manner. In addition, plasma levels of reactive oxygen species (ROS) increased in rats administered acrylonitrile. Dietary supplementation with NAC prevented acrylonitrile-induced oxidative DNA damage in brain and WBC. A slight, but significant, decrease in the GSH:GSSG ratio was seen in brain at acrylonitrile doses > 30 ppm. These results provide additional support that the mode of action for acrylonitrile-induced astrocytomas involves the induction of oxidative stress and damage. Significant associations were seen between oxidative DNA damage in WBC and brain, ROS formation in plasma, and the reported tumor incidences. Since oxidative DNA damage in brain correlated with oxidative damage in WBC, these results suggest that monitoring WBC DNA damage maybe a useful tool to assess acrylonitrile-induced oxidative stress in humans.
Keywords: acrylonitrile, comet assay, oxidative DNA damage, brain, white blood cells
Acrylonitrile is widely used in the manufacture of plastics, acrylic fibers, and synthetic rubber with an estimated yearly production of 8.8 billion pounds (IARC, 1999; Nerland et al., 2003). Chronic exposure to acrylonitrile caused dose-related increases in brain tumors in rats (Bigner et al., 1986; Bio/dynamics Inc., 1980; Frederick and Levinskas, 2002; Quast and Friedman, 2002; Quast et al., 1980). Evaluation of epidemiological data on the cancer incidences in workers exposed to acrylonitrile has found no consistent increase in cancer risk from acrylonitrile exposure (Cole et al., 2008).
The mechanism of acrylonitrile carcinogenicity in the rat brain is not fully understood. The available data from both in vivo and in vitro genotoxicity tests, while largely negative, are mixed and inconclusive (IARC, 1999; Whysner et al., 1998b). Acrylonitrile is metabolized by pathways involving glutathione conjugation and cytochrome P450 oxidation (Abreu and Ahmed, 1980; Chanas et al., 2003; Fennell et al., 1991; Kedderis et al., 1995; Roberts et al., 1991). Cytochrome P450 metabolism produces 2-cyanothylene oxide (CEO), a reactive and relatively long-living epoxide (Guengerich et al., 1981; Kedderis et al., 1995; Roberts et al., 1991). While this metabolite forms DNA adducts in rat liver, a nontumor target organ for acrylonitrile carcinogenicity, DNA reactivity does not appear to explain acrylonitrile-induced brain tumors since no DNA adducts have been detected in rat brain (Hogy and Guengerich, 1986).
In recent years, several studies have demonstrated that acrylonitrile causes oxidative stress and oxidative DNA damage in the rat brain and cultured rat astrocytes (El-Sayed et al., 2008; Jacob and Ahmed, 2003; Jiang et al., 1998; Kamendulis et al., 1999; Pu et al., 2006; Whysner et al., 1998a). Increased oxidative stress and oxidative damage have been linked with the induction of neoplasia by several nongenotoxic chemical carcinogens (Kensler and Thrush, 1986; Kensler et al., 1989; Klaunig and Kamendulis, 2004). Oxidative stress may be involved in carcinogenesis through the depletion of antioxidants and/or the increased production of reactive oxygen species (ROS), the latter resulting in modifications to cellular DNA. In addition, low levels of cellular oxidants can function as signaling molecules that activate transcription factors impacting pathways involved in cell growth (Klaunig and Kamendulis, 2004). Acrylonitrile has been shown to affect both antioxidant and ROS levels following exposure (Jiang et al., 1998; Kamendulis et al., 1999). In the rat brain in vivo, acrylonitrile increased levels of 8′-hydroxyl-2-deoxyguanosine (OH8dG), malondialdehyde, and ROS and decreased levels of glutathione and activities of catalase and superoxide dismutase (El-Sayed et al., 2008; Jiang et al., 1998; Whysner et al., 1998a). In a rat astrocyte cell line, acrylonitrile similarly increased OH8dG levels and the formation of hydroxyl free radicals (Kamendulis et al., 1999). Acrylonitrile also induced oxidative stress in normal human astrocytes in vitro, albeit at concentrations that produced significant cytolethality (Jacob and Ahmed, 2003). In a more recent study, acrylonitrile increased oxidative DNA damage in rat astrocytes while was unable to directly cause DNA damage (Pu et al., 2006). In addition, the antioxidant N-acetyl cysteine (NAC) was shown to protect primary rat glial cells against lipid peroxidation and GSH depletion induced by acrylonitrile (Esmat et al., 2007). Collectively, these data support that oxidative stress occurs following acrylonitrile exposure and may potentially be the mode of action for glial tumor formation by acrylonitrile.
In animal studies, the measurement of OH8dG by high-performance liquid chromatography (HPLC) with electrochemical detection has been widely used as a marker for oxidative DNA damage. Although this technique is very sensitive and well defined, it is inherently impractical to be used in human studies on brain tissue samples. Thus, surrogate techniques are needed to assess oxidative stress in humans at risk from acrylonitrile exposure. The present study examined the dose-related effects of acrylonitrile on the formation of ROS, glutathione modulation, and oxidative DNA damage in rat brain and blood in vivo. The concordance between the oxidative stress and the damage measurements in these tissues and the tumor incidence reported in the literature was also analyzed to assess the potential feasibility of using these oxidative stress biomarkers in monitoring oxidative stress in humans at risk from exposure to acrylonitrile.
MATERIALS AND METHODS
Chemicals.
Acrylonitrile (> 99% purity), 2-deoxyguanosine (2-dG), 8′-hydroxyl-2-deoxyguanosine, reduced and oxidized glutathione (GSH and GSSG), salicylic acid (SA), 2,3-dihydroxybenzoic acid (2,3-DHBA), and all other chemicals were purchased from Aldrich (Milwaukee, WI). Formamidopyrimidine DNA glycosylase (fpg) was purchased from New England Biolab (Ipswich, MA). NIH-07-based diets containing 0.3% NAC was purchased from Dyets Inc. (Bethlehem, PA).
Animals and treatments.
Six-week-old male Sprague-Dawley rats were purchased from Harlan Sprague-Dawley Co. (Indianapolis, IN). Rats were housed in polycarbonate cages in an Association for Assessment and Accreditation of Laboratory Animal Care-certified animal facility and provided with an NIH-07 diet and water ad libitum. Animals were allowed to acclimate for 1 week prior to the experiments. Animals were given 0, 3, 30, 100, and 200 ppm in deionized drinking water for 28 days. These doses were selected since these were the doses used in the chronic 2-year bioassays. To examine the effects of acrylonitrile exposure on the formation of ROS, three rats in each group were administered SA in 0.9% NaCl solution (50 mg/kg body weight) by ip injection 12 h before the sacrifice. To examine the effect of NAC on acrylonitrile-induced oxidative stress and damage, animals were given diet containing 0.3% NAC 3 days prior to and through out acrylonitrile treatment (200 ppm in drinking water) for 28 days. At the end of treatments, rats were killed by CO2 asphyxiation and exsanguination. Blood samples were collected immediately by cardiopuncture in plastic tubes containing Na2EDTA. After an aliquot of blood sample was obtained for comet assay, plasma was separated by centrifugation at 4°C and stored at −80°C for subsequent analysis.
Assessment of direct and oxidative DNA damage—comet assay.
The comet assay was performed as described previously with modifications (Rojas et al., 1999; Singh et al., 1988). For white blood cells (WBC), immediately after the blood samples were taken, 2 μl whole blood was mixed with 70 μl 1% low-melting agarose and applied onto Trevigen CometSlides (Trevigen, Gaithersburg, MD). For the analysis of DNA damage in the brain, immediately after sacrifice, ∼200 mg of brain cortex was washed then minced in cold PBS containing 10% dimethyl sulfoxide (DMSO) and 1mM EDTA, filtered (40-μm filter), and centrifuged (at 200 × g for 5 min at 4°C). The pellets were resuspended in cold PBS, and a 10-μl aliquot was mixed with 70 μl 1% low-melting agarose and applied onto Trevigen CometSlides. After the gel solidified, cells were lysed (100mM Na2EDTA, pH 10, 2.5M NaCl, 10mM Trizma base, 1% sodium lauryl sarcosinate, 1% Triton-X 100, 10% DMSO, and 1mM deferoxamine; 1 h), placed in alkali buffer (0.3M NaOH and 1mM Na2EDTA, pH > 13; for 40 min at 4°C), and then electrophoresed in alkali buffer (25 V, 300 mA, 30 min). Following electrophoresis, slides were neutralized (0.4M Tris, pH 7.5, at 4°C for 5 min), rinsed in distilled water (at 4°C for 15 min), and allowed to dry at room temperature in the dark. After slides were stained with ethidium bromide (25 μg; 20 μg/ml) and covered with a cover slip, a total of 100 nuclei/treatment selected at random per slide were evaluated on a Nikon fluorescence microscope using Komet 4.0 imaging Software (Kinetic Imaging Ltd., Liverpool, UK). DNA damage was expressed as comet (Olive) tail moment ([tail mean − head mean] × tail%DNA/100) (Rojas et al., 1999; Tice et al., 1991).
For the assessment of oxidative DNA damage, a modified alkaline comet assay was performed that included enzymatic digestion with fpg prior to electrophoresis. Briefly, after the lysis of cells, slides were washed (40mM 4-[2-hydroxyethyl]piperazine-1-ethanesulfonic acid, 100mM KCl, 0.5mM Na2EDTA, and 0.2% bovine serum albumin; pH 8.0, 5 min, 3×) and then incubated with fpg solution or buffer (100 μl for 40 min at 37°C). Slides then underwent alkaline unwinding and the remainder of the procedure as described above.
Measurement of OH8dG.
OH8dG levels in genomic DNA in brain tissues and WBC were analyzed by HPLC with electrochemical detection as described by Helbock (1999) with modifications. Deferoxamine (0.1mM), an iron chelator, was added to all reagents during DNA isolation and hydrolysis to minimize DNA autooxidation. For brain tissues, 100 mg of tissue was homogenized in 1 ml of homogenization buffer (0.32M sucrose, 5mM MgCl2, 10mM Tris-HCl, pH 7.5, and 0.1mM mesylate). The homogenate was then centrifuged at 900 × g for 5 min at 4°C. The pellets were resuspended in 1 ml lysis buffer (homogenization buffer containing 1% Triton-X 100, pH 7.5) and centrifuged at 900 × g for 1 min at 4°C. This step was repeated twice. The pellets were then thoroughly resuspended in 200 μl enzyme reaction buffer (1% SDS, 5mM Na2EDTA, and 10mM Tris-HCl, pH 8.0) and digested with 5 U of RNase (at 50°C for 10 min), followed by the digestion with Proteinase K (15 mg/ml, at 50°C for 50 min). After digestion, 0.3 ml of NaI solution (7.6M NaI, 20mM Na2EDTA, and 40mM Tris-HCl, pH 8.0) was added and DNA was then precipitated with 0.5 ml of isopropanol. DNA was pelleted by centrifugation (12,000× g for 5 min) and washed with 70% ethanol. For the WBC, 5 ml whole blood from rats was treated with red blood cell lysis buffer (80mM NH4Cl) and centrifuged. Pelleted WBC were then lysed with lysis buffer, and the remainder of the procedure described for brain tissue (above) was followed. DNA was dissolved in 200 μl of 20mM sodium acetate containing 0.1mM deferoxamine, pH 4.8, and digested with 10 U of nuclease P1 (at 65°C for 30 min), followed by 10 U of alkaline phosphatase (at 37°C for 60 min). Digested DNA samples were filtered and an 80-μl aliquot was injected into HPLC system. Nucleotides were separated on a reverse phase LC-18-DB column (3μM, 150 × 4.6 mm; Supelco, Bellefonte, PA). OH8dG was detected by an ESA CoulArray electrochemical detector set at 400 mV, and 2-dG was detected by a Waters photodiode array detector set at 290 nm. OH8dG and 2-dG in samples were quantitated from respective calibration curves, and the ratio of OH8dG:2-dG in each sample was reported.
Measurement of ROS production.
The formation of ROS in plasma from acrylonitrile-treated rats (pretreated with SA) was measured using the method described previously (McCabe et al., 1997). Briefly, 200-μl plasma sample was mixed with 800 μl 0.2M perchloric acid solution (containing 100μM EDTA and 100μM sodium metabisulfide) to precipitate protein. After centrifugation (14,000 × g at 4°C for 5 min), the supernatant was filtered and an 80-μl aliquot was injected onto the HPLC. Separation of analytes was achieved on a reverse phase YMC J'sphere ODS-H80 column (4 μm, 150 × 4.6 mm; Waters, Milford, MA). The 2,3-DHBA was detected on an ESA Coulochem II equipped with a guard cell. The potential settings for the detector were E1 100 mV, E2 250 mV, and guard cell 1000 mV. SA was detected by a Waters Photodiode Array Detector set at 296 nm. The amounts of 2,3-DHBA and SA were quantitated from respective calibration curves, and the ratio of 2,3-DHBA:SA in each sample was reported.
Measurement of reduced and oxidized glutathione.
Reduced and oxidized glutathione (GSH and GSSG) in brain tissues were analyzed simultaneously using HPLC-electrochemical detection as described previously (Melnyk et al., 1999). Briefly, 200 mg of brain tissue was homogenized in 1 ml of buffer containing 0.2M perchloric acid and 100μM EDTA. The homogenate was centrifuged (14,000 × g at 4°C for 5 min). Supernatants were filtered and injected into HPLC for analysis. The separation of analytes was achieved on a reverse phase Symmetry C-18 column (5 μm, 150 × 4.6 mm; Waters). GSH and GSSG were detected using an ESA Coulochem II equipped with a guard cell. The potential settings for the detector were E1 450 mV, E2 900 mV, and guard cell 1000 mV. The amount of GSH and GSSG was calculated from the respective calibration curves.
Statistical analysis.
The values were presented as mean ± SD. Differences among multiple groups were evaluated using a one-way ANOVA with Dunnett’s post hoc test. The level of significance was selected to be p < 0.05.
RESULTS
All rats survived the treatment period. No statistical differences in body weights were seen among the treatment groups received drinking water containing ≤100 ppm acrylonitrile for 28 days (data not shown). However, a significant decrease (8.3%) in the body weight was observed in animals treated with 200 ppm acrylonitrile compared to control.
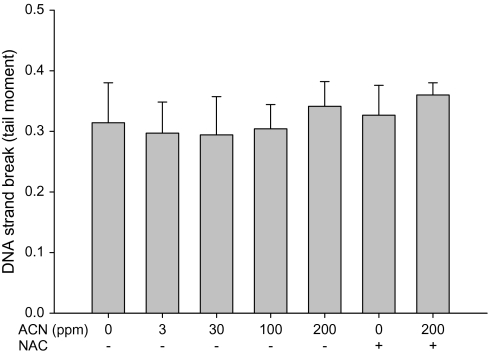
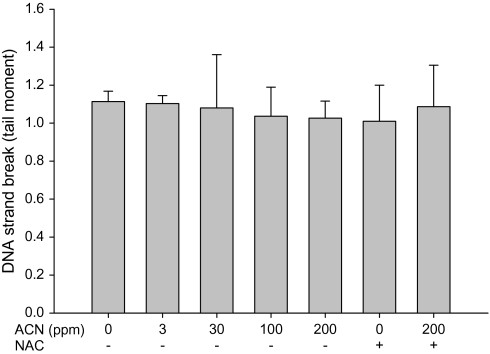
The ability of acrylonitrile to induce direct DNA strand breakage in the WBC and brain cortex in rats was examined using the alkaline comet assay. No significant increase in direct DNA strand breakage was found in either tissue following the treatment with 0–200 ppm acrylonitrile in drinking water for 28 days (Figs. 1 and 2), indicating that acrylonitrile does not cause direct DNA damage in brain and WBC.
FIG. 1.
Direct DNA damage in rat WBC following exposure to acrylonitrile (ACN) in drinking water ± NAC in diet for 28 days measured by alkaline comet assay. Values represent the means ± SD in nine animals.
FIG. 2.
Direct DNA damage in rat brain following exposure to acrylonitrile (ACN) in drinking water ± NAC in diet for 28 days measured by alkaline comet assay. Values represent the means ± SD in three animals.
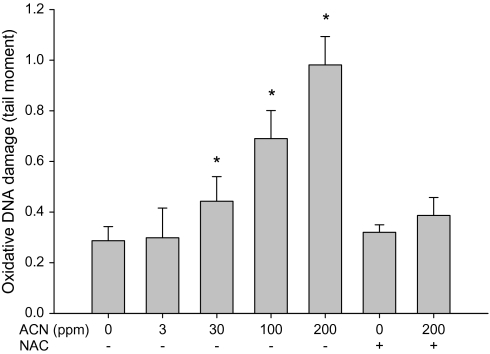
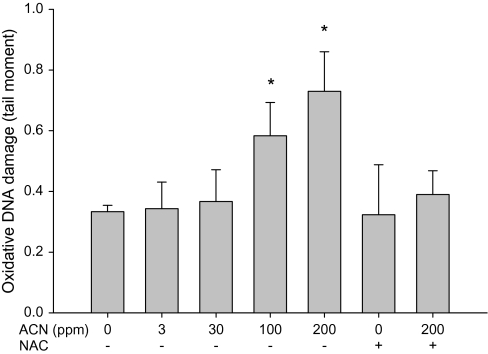
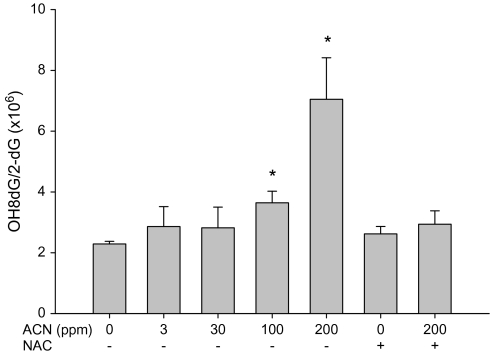
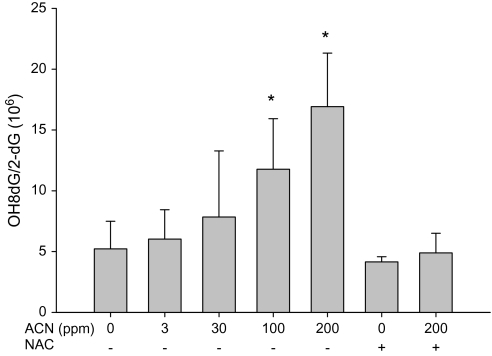
The ability of acrylonitrile to induce oxidative DNA damage in brain and WBC of rats was examined using both fpg-modified comet assay and by measuring OH8dG levels (HPLC-electrochemical detection). Increases in oxidative DNA damage in WBC were seen following the exposure with 30, 100, and 200 ppm acrylonitrile in drinking water (Fig. 3). Increases in oxidative DNA in cells from brain cortex were also observed following the exposure with 100 and 200 ppm acrylonitrile in drinking water (Fig. 4). Supplementation of NAC in diets prevented the oxidative DNA damage in brain and WBC induced by acrylonitrile (Figs. 3 and 4). OH8dG levels in the brain and WBC were significantly increased in animals treated with 100 and 200 ppm acrylonitrile in drinking water for 28 days (Figs. 5 and 6). NAC supplementation also prevented acrylonitrile-induced OH8dG formation (Figs. 5 and 6).
FIG. 3.
Oxidative DNA damage in rat WBC following exposure to acrylonitrile (ACN) in drinking water ± NAC in diet for 28 days measured by fpg-modified alkaline comet assay. Values represent the means ± SD in nine animals. *Statistically different from control (p < 0.05, ANOVA with Dunnett’s post hoc test).
FIG. 4.
Oxidative DNA damage in rat brain following exposure to acrylonitrile (ACN) in drinking water ± NAC in diet for 28 days measured by fpg-modified alkaline comet assay. Values represent the means ± SD in three animals. *Statistically different from control (p < 0.05, ANOVA with Dunnett’s post hoc test).
FIG. 5.
OH8dG level in rat WBC following the exposure to acrylonitrile (ACN) in drinking water ± NAC for 28 days. Values represent the means ± SD in three animals. *Statistically different from respective control (p < 0.05, ANOVA with Dunnett's post hoc test).
FIG. 6.
OH8dG level in rat brain following the exposure with acrylonitrile (ACN) in drinking water ± NAC for 28 days. Values represent the means ± SD in nine animals. *Statistically different from respective control (p < 0.05, ANOVA with Dunnett's post hoc test).
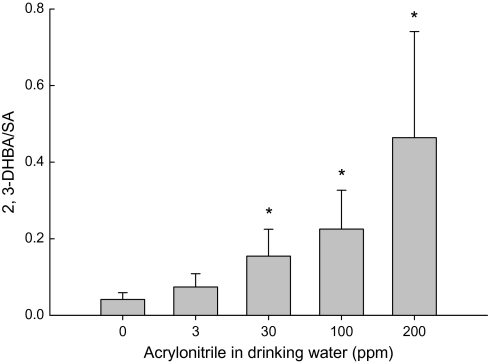
Salicylate can react with the hydroxyl free radical, a strong ROS, to form 2,3-DHBA, a stable product that can be quantitated using HPLC with electrochemical detection (McCabe et al., 1997). The generation of ROS in rat plasma following the administration of acrylonitrile in drinking water was examined. Significant and dose-related elevation of the levels of 2,3-DHBA was observed in the plasma of rats at and above 30 ppm acrylonitrile in drinking water for 28 days, indicating that acrylonitrile exposure increased the generation of ROS rats (Fig. 7).
FIG. 7.
Effect of acrylonitrile (ACN) on the formation of ROS in rat plasma following the exposure in drinking water for 28 days. Values represent the means ± SD in three animals. *Statistically different from respective control (p < 0.05, ANOVA with Dunnett's post hoc test).
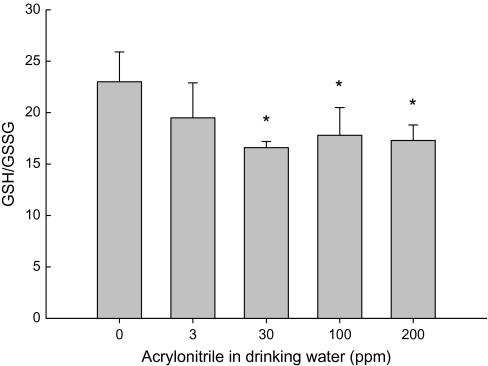
The ratio of GSH:GSSG is an important indicator of the redox status in the biological systems. GSH and GSSG levels in brain tissues were measured simultaneously using HPLC-electrochemical detection. No significant changes in GSH level were seen in any acrylonitrile-treated groups (data not shown). However, a slight but significant decrease in the GSH/GSSG was observed in brain cortexes of rats administered 30, 100 and 200 ppm acrylonitrile in drinking water for 28 days (Fig. 8).
FIG. 8.
Effect of acrylonitrile (ACN) on GSG/GSSG in rat brain cortexes following the exposure in drinking water for 28 days. Values represent the means ± SD in six observations. *Statistically different from control (p < 0.05, ANOVA with Dunnett's post hoc test).
The concordance between the oxidative stress and the damage measurements in brain and blood and between oxidative damage and tumor incidence reported in the literature was analyzed (Table 1). Strong positive correlations between the oxidative DNA damage measured by fpg-modified comet and HPLC (OH8dG formation) in brain and WBC were observed. There were also significant correlations between these oxidative markers and the reported brain tumor incidence (Table 1). These results suggest that these oxidative stress biomarkers in blood could be utilized in monitoring oxidative stress in humans at risk from exposure to acrylonitrile.
TABLE 1.
Concordance Between Oxidative Stress and Damage Measurements in Brain and WBC and Reported Tumor Incidence in Sprauge-Dawley Rats.
| Correlations | R2 |
| GSH/GSSG in brain vs. OH8dG level in brain | 0.36 |
| OH8dG level in brain vs. OH8dG level in WBC | 0.90* |
| Oxidative DNA damage (fpg comet) in brain vs. oxidative DNA damage (fpg comet) in WBC | 0.91* |
| Oxidative DNA damage (fpg comet) in WBC vs. ROS in plasma | 0.98* |
| OH8dG level in brain vs. ROS in plasma | 0.97* |
| Adjusted OH8dG level in braina vs. tumor incidenceb | 0.92* |
| Adjusted oxidative DNA damage in brain (comet assay)a vs. tumor incidenceb | 0.93* |
| Adjusted OH8dG level in WBCa vs. tumor incidenceb | 0.82* |
| Adjusted oxidative DNA damage in WBC (comet assay)a vs. tumor incidenceb | 0.90* |
Note. Data were analyzed by linear regression. *Statistically significant (p < 0.05, ANOVA).
Data were normalized with doses used in tumor incidence bioassay.
Tumor incidence data were from Quast and Friedman (2002).
DISCUSSION
It is well established that acrylonitrile is a carcinogen in rats, including brain tumors (astrocytomas), following chronic exposure (Bigner et al., 1986; Bio/dynamics Inc., 1980; Frederick and Levinskas, 2002; Quast and Friedman, 2002; Quast et al., 1980). The mechanism(s) of acrylonitrile tumorigenicity have not been conclusively demonstrated; however, a substantial body of evidence exists supporting the induction of oxidative stress as the mode of action for astrocytoma induction in rats (Jacob and Ahmed, 2003; Jiang et al., 1998; Kamendulis et al., 1999; Pu et al., 2006; Whysner et al., 1998a). Oxidative stress is defined as a disturbance in the prooxidant to antioxidant balance, in favor of the former (Sies, 1991). Oxidative stress and damage have been shown to participate at all stages of chemical carcinogenesis (Klaunig and Kamendulis, 2004).
Acrylonitrile is metabolized to CEO, a relatively stable and reactive epoxide. Chemical adducts have only been seen in liver homogenates at high dose, while no studies have identified acrylonitrile adducts in rat brain (Guengerich et al., 1981; Hogy and Guengerich, 1986; Kedderis et al., 1995; Roberts et al., 1991). Due to the reactivity of CEO, the potential for acrylonitrile to induce DNA damage in the rat brain, an event that may contribute to carcinogenicity, continues to be raised. A recent study demonstrated that acrylonitrile did not directly damage DNA of astrocytes but did result in increased oxidative DNA damage (Pu et al., 2006). This study also demonstrated that the oxidative DNA damage seen following acrylonitrile exposure appears to arise mainly through the P450 metabolic pathway and that glutathione depletion may contribute to the induction of oxidative DNA damage (Pu et al., 2006).
The current study further examined the effects of acrylonitrile on oxidative stress by assessing oxidative DNA damage, formation of ROS, and glutathione modulation in rats in both blood and brain tissue. The potential direct DNA damage by acrylonitrile in WBC and brain was assessed using single cell gel electrophoresis (the comet assay). Acrylonitrile was administered up to and including 200 ppm in drinking water to rats and did not result in DNA strand breakage in brain or WBC. Previous studies demonstrated that acrylonitrile induced oxidative stress in rat brain in vivo and astrocytes in vitro (Jacob and Ahmed, 2003; Jiang et al., 1998; Kamendulis et al., 1999; Pu et al., 2006; Whysner et al., 1998a). The results of the current investigation are consistent with these previous studies. We demonstrate that acrylonitrile induced oxidative DNA damage in rat brain and WBC in a dose-dependent manner following oral exposure in drinking water as measured by both the fpg-modified comet assay and OH8dG quantitation using HPLC-electrochemical detection. The concordance analysis showed strong correlations between OH8dG levels in brain and WBC and oxidative DNA damage in these tissues measured by fpg-modified alkaline comet assay, indicating that comet assay in WBC could be used as surrogate test to assess acrylonitrile-induced oxidative stress in humans (Table 1).
The formation of ROS in the rat plasma was measured using HPLC-electrochemical detection of salicylate hydroxylation. The results of this study showed a dose-dependent increase in the level of 2,3-DHBA in the plasma of rats treated with acrylonitrile in drinking water, indicating that acrylonitrile exposure resulted in the generation of ROS. Significant correlations were observed between ROS in plasma and DNA damage in brain as well as ROS in plasma and oxidative DNA damage in WBC (Table 1). These results also demonstrate that the oxidative stress biomarkers measured in this study are similarly changed by acrylonitrile exposure, further supporting that fpg-modified comet assay in WBC could serve as a marker for the assessment of oxidative stress in human populations exposed to acrylonitrile. In a previous study, a dose-dependent increase of 2,3-DHBA in rat brain was found (Jiang et al., 1998). However, 2,3-DHBA was not detectable in brain in the current study. One possible reason for this discrepancy might be that in the current study, animals received lower dose of SA than the previous study (50 vs. 100 mg/kg), a change that was made to avoid the potential interference that salicylate, a scavenger of ROS, may have on the comet assay in blood. Nonetheless, the findings in the current and previous studies provide evidence that acrylonitrile induces oxidative stress.
ROS are produced by both endogenous and exogenous processes. Eukaryotic organisms have developed a complex antioxidant system to counteract excess ROS production and to reduce the deleterious effects of excess oxidant production. Glutathione is an abundant water-soluble thiol-containing tripeptide, making this molecule a potent reducing agent. GSH can be converted to GSSG upon the reaction with ROS. Of potential relevance to acrylonitrile-induced oxidative stress and tumorigenicity, the rat brain has been shown to possess a lower antioxidant capacity relative to the liver (Jiang et al., 1998; Xia et al., 1995). Acrylonitrile has been shown to induce a temporal depletion of GSH in rat glial cells in culture and in rat brain in vivo (decreased by 7 days and restored to control levels by 28 days) (Kamendulis et al., 1999). The present study showed similar effects. GSH was not decreased in rat brain by acrylonitrile at 28 days. However, a slight but significant decrease in GSH/GSSG was observed in rats administered with ≥30 ppm acrylonitrile in drinking water at 28-day time point. GSH/GSSG is an important indicator of redox status in a biological system. The decrease of the GSH:GSSG ratio indicates that redox balance shifted in favor of prooxidant status, which contributes to the overall oxidative stress status and may result in the oxidative damage to macromolecules in cells.
NAC is an acetylated precursor of glutathione, a major water-soluble antioxidant in cells. NAC has been reported to protect primary rat glial cells against lipid peroxidation and GSH depletion induced by acrylonitrile (Esmat et al., 2007). Similar protective effects of NAC were observed in this study. Dietary supplementation of NAC prevented acrylonitrile-induced oxidative DNA damage in brain and WBC. These results provide additional evidence that acrylonitrile induces oxidative stress in rats.
HPLC with electrochemical detection of OH8dG has been used in the measurement of acrylonitrile-induced DNA damage in brain tissues. Although this technique is very sensitive and well characterized, it is inherently impractical to be used to monitor oxidative DNA damage in brains of live human beings. Thus, surrogate techniques are needed to assess oxidative stress in acrylonitrile-exposed humans. The assessment of DNA damage using comet assay provides a simple, sensitive, and straightforward approach to directly quantitate DNA damage in cells (Singh et al., 1988). In addition, the incorporation of enzymatic digestion with fpg enables the detection of oxidative DNA damage, including 8-oxoguanine and other oxidized purines (Collins, 2004). Comet assay and fpg-modified comet assay in WBC have been commonly used in investigating DNA damage and oxidative DNA damage in human studies (Dennog et al., 1999; Speit et al., 2002). In this study, the fpg-modified comet assay was used to measure the oxidative DNA damage in brain and WBC in rats, and as discussed above, significant correlations were seen between the oxidative DNA damage in brain and WBC assessed by fpg-modified comet assay and OH8dG detected by HPLC, as well as the ROS levels in plasma (Table 1). This indicates that it is feasible to use comet assay in WBC as a means to monitor potential oxidative stress and damage from acrylonitrile exposure in humans.
The concordance between the oxidative DNA damage in WBC measured by fpg-modified comet assay of current study and the tumor incidence in central nervous system after acrylonitrile exposure reported in literature was analyzed (Table 1). The tumor incidence data used in the analysis were from a 2-year carcinogenicity bioassay by Quast and Friedman (2002) in which the same strain of rats (Sprague-Dawley) and similar doses were investigated. Oxidative DNA damage in brain and WBC measured by both HPLC and fpg-modified alkaline comet assay significantly correlated with tumor incidences in rat brain (Table 1). The positive associations between comet assay data, OH8dG levels, and tumor incidences provide further support that induction of oxidative stress is involved in the mode of action for acrylonitrile carcinogenicity.
In summary, the results presented in this study have demonstrated that acrylonitrile did not induce detectable direct DNA strand breakage, but using coincubation with fpg, as well as HPLC-electrochemical detection, we confirmed that acrylonitrile induces oxidative stress and oxidative DNA damage in rats in a dose-dependent manner and was prevented by the dietary supplementation with antioxidant NAC. These results provide additional support that the mode of action for acrylonitrile-induced brain tumor involves the induction of oxidative stress and damage. Furthermore, this study demonstrated that fpg-modified comet assay in WBC correlated with oxidative DNA damage in acrylonitrile target tissue and therefore may be a useful tool to assess acrylonitrile-induced oxidative stress in humans at exposure.
FUNDING
Supported in part by grants from National Institute of Health/National Cancer Institute (R01-CA100908, J.E.K.); The Acrylonitrile Group Inc.
References
- Abreu ME, Ahmed AE. Metabolism of acrylonitrile to cyanide: In vitro studies. Drug. Metab. Dispos. 1980;8:376–379. [PubMed] [Google Scholar]
- Bigner DD, Bigner SH, Burger JD, Shelburne TJD, Friedman HS. Primary brain tumors in Fisher 344 rats chronically exposed to acrylonitrile in their drinking-water. Food Chem. Toxicol. 1986;24:129–137. doi: 10.1016/0278-6915(86)90347-9. [DOI] [PubMed] [Google Scholar]
- Bio/dynamics Inc. A Twenty-our Month Oral Toxicity/Carcinogenicity Study of Acrylonitrile Administered in Drinking Water to Fisher 344 Rats. 1980. Final Report, Vol. I. Monsanto Company, St. Louis, MO. [Google Scholar]
- Chanas B, Wang H, Ghanayem BI. Differential metabolism of acrylonitrile to cyanide is responsible for the greater sensitivity of male vs female mice: Role of CPY2E1 and epoxide hydrolases. Toxicol. Appl. Pharm. 2003;193:293–302. doi: 10.1016/j.taap.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Cole P, Mandel JS, Collins JJ. Acrylonitrile and cancer: A review of the epidemiology. Regul. Toxicol. Pharmacol. 2008;52:342–351. doi: 10.1016/j.yrtph.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Collins AR. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotech. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- Dennog C, Gedik C, Wood S, Speit G. Analysis of oxidative DNA damage and HPRT mutations in humans after hyperbaric oxygen treatment. Mutat. Res. 1999;431:351–359. doi: 10.1016/s0027-5107(99)00178-5. [DOI] [PubMed] [Google Scholar]
- El-Sayed el-SM, Abo-Salem OM, Abd-Ellah MF, Abd-Alla GM. Hesperidin, an antioxidant flavonoid, prevents acrylonitrile-induced oxidative stress in rat brain. J. Biochem. Mol. Toxicol. 2008;22:268–273. doi: 10.1002/jbt.20237. [DOI] [PubMed] [Google Scholar]
- Esmat A, El-Demerdash E, El-Mesallamy H, Abdel-Naim AB. Toxicity and oxidative stress of acrylonitrile in rat primary glial cells: Preventive effects N-acetylcysteine. Toxicol. Lett. 2007;171:111–118. doi: 10.1016/j.toxlet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Fennell TR, Kedderis GL, Sumner SC. Urinary metabolites of (1,2,3-13C acrylonitrile in rats and mice detected by 13C nuclear magnetic resonance spectroscopy. Chem. Res. Toxicol. 1991;4:678–687. doi: 10.1021/tx00024a013. [DOI] [PubMed] [Google Scholar]
- Frederick FJ, Levinskas GJ. Chronic toxicity and oncogenic dose-response effects of lifetime oral acrylonitrile exposure to Fisher 344 rats. Toxicol. Lett. 2002;132:221–247. doi: 10.1016/s0378-4274(02)00074-7. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Geiger LE, Hogy LL, Wright PL. In vitro metabolism of acrylonitrile to 2-cyanoethylene oxide, reaction with glutathione, and irreversible binding to proteins and nucleic acid. Cancer Res. 1981;41:4925–4933. [PubMed] [Google Scholar]
- Helbock HJ, Bechman KB, Ames B. 8-hydroxydeoxyguanosine and 8-Hydroxyguanine as biomarkers of oxidative DNA damage. Methods Enzymol. 1999;300:156–166. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- Hogy LL, Guengerich FP. In vitro interaction of acrylonitrile and 2-cyanoethylene oxide with DNA in rats. Cancer Res. 1986;46:3932–3938. [PubMed] [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans. Lyon, France: International Agency for Research on Cancer; 1999. [Google Scholar]
- Jacob S, Ahmed AE. Acrylonitrile-induced neurotoxicity in normal human astrocytes: Oxidative stress and 8-hydroxy-2′-deoxyguanosine formation. Toxicol. Mech. Method. 2003;13:169–179. doi: 10.1080/15376510309836. [DOI] [PubMed] [Google Scholar]
- Jiang J, Xu Y, Klaunig JE. Induction of oxidative stress in rat brain by acrylonitrile. Toxicol. Sci. 1998;46:333–341. doi: 10.1006/toxs.1998.2524. [DOI] [PubMed] [Google Scholar]
- Kamendulis LM, Jiang J, Xu Y, Klaunig JE. Induction of oxidative stress and oxidative damage in rat glial cells by acrylonitrile. Carcinogenesis. 1999;20:1555–1560. doi: 10.1093/carcin/20.8.1555. [DOI] [PubMed] [Google Scholar]
- Kedderis GL, Batra R, Turner MJ., Jr Conjugation of acrylonitrile and 2-cyanoethylene oxide with hepatic glutathione. Toxicol. Appl. Pharm. 1995;135:9–17. doi: 10.1006/taap.1995.1203. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Taffe BG, Thrush MA. Role of free radicals in tumor promotion and progression. Prog. Clin. Biol. Res. 1989;298:233–248. [PubMed] [Google Scholar]
- Kensler TW, Thrush MA. Role of oxygen radicals in tumor promotion. Environ. Mutagen. 1986;6:593–616. doi: 10.1002/em.2860060412. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- McCabe DR, Maher TJ, Acworth IN. Improved method for the estimation of hydroxyl free radical levels in vivo based on liquid chromatography with electrochemical detection. J. Chromatogr. B: Biomed. Sci. Appl. 1997;691:23–32. doi: 10.1016/s0378-4347(96)00416-1. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Progribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneously determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J. Nutr. Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Nerland DE, Cai J, Benz FW. Selective covalent binding of acrylonitrile to cys 186 in rat liver carbonic anhydrase III in vivo. Chem. Res. Toxicol. 2003;16:583–589. doi: 10.1021/tx0256883. [DOI] [PubMed] [Google Scholar]
- Pu X, Kamendulis LM, Klaunig JE. Acrylonitrile-induced oxidative DNA damage in rat astrocytes. J. Environ. Mol. Mutagen. 2006;47:631–638. doi: 10.1002/em.20249. [DOI] [PubMed] [Google Scholar]
- Quast JF, Friedman MA. Two-year toxicity and oncogenicity study with acrylonitrile incorporated in drinking water of rats. Toxicol. Lett. 2002;132:153–196. doi: 10.1016/s0378-4274(02)00072-3. [DOI] [PubMed] [Google Scholar]
- Quast JF, Wade CE, Humiston CG, Gushow TS, Park CN, Mckenna MJ. A Two-year Toxicity and Oncogenicity Study with Acrylonitrile Incorporated in Drinking Water of Rats. Midland, MI: Dow Chemical Co.; 1980. [Google Scholar]
- Roberts AE, Kedderis GL, Turner MJ, Rickert DE, Swenberg JA. Species comparison of acrylonitrile epoxidation by microsomes from mice, rats and humans: Relationship to epoxide concentrations in mouse and rat blood. Carcinogenesis. 1991;12:401–404. doi: 10.1093/carcin/12.3.401. [DOI] [PubMed] [Google Scholar]
- Rojas E, Lopez MC, Valverde M. Single cell gel electrophoresis assay: Methodology and applications. J. Chromatogr. B: Biomed. Sci. Appl. 1999;722:225–254. doi: 10.1016/s0378-4347(98)00313-2. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991;91:31s–38s. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- Singh PN, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Speit G, Schutz P, Trenz K, Rothfuss A. Oxygenated water does not induce genotoxic effects in comet assay. Toxicol. Lett. 2002;133:203–210. doi: 10.1016/s0378-4274(02)00153-4. [DOI] [PubMed] [Google Scholar]
- Tice RR, Andrews PW, Hirai O, Singh NP. The single cell gel (SCG) assay: An electrophoretic technique for the detection of DNA damage in individual cells. Adv. Exp. Med. Biol. 1991;283:157–164. doi: 10.1007/978-1-4684-5877-0_17. [DOI] [PubMed] [Google Scholar]
- Whysner J, Robert ES, Chen D, Conaway CC, Verna LK, Richie JP, Ali N, Williams GM. Formation of 8-oxodeoxyguanosine in brain DNA of rats exposed to acrylonitrile. Arch. Toxicol. 1998a;72:429–438. doi: 10.1007/s002040050523. [DOI] [PubMed] [Google Scholar]
- Whysner J, Ross PM, Conaway CC, Verna LK, Williams GM. Evaluation of possible genotoxic mechanisms for acrylonitrile tumorigenicity. Regul. Toxicol. Pharm. 1998b;27:217–239. doi: 10.1006/rtph.1998.1204. [DOI] [PubMed] [Google Scholar]
- Xia E, Rao G, Remmen HV, Heydari AR, Richardson A. Activities of antioxidant enzymes in various tissues of male Fisher 344 rats are altered by food restriction. J. Nutr. 1995;125:195–201. doi: 10.1093/jn/125.2.195. [DOI] [PubMed] [Google Scholar]