Abstract
Background: One of the most enduring and replicated findings in biological psychiatry is activation of the hypothalamic-pituitary-adrenal (HPA) axis in a subset of patients with major depressive disorder. This review will discuss some of these findings and their pertinence to the assessment and treatment of depressed patients.
Method: MEDLINE, PsychINFO, and Current Contents databases were searched for pertinent articles on the HPA axis in patients with depression. In addition, hand searches were conducted of references from these sources and abstracts from meetings and books on this topic. Articles that would provide an overview of major or interesting studies in the field were selected for inclusion.
Results: The data support that HPA axis activation is common in depressed patients. Frequently reported findings include elevated cortisol and corticotropin-releasing hormone (CRH), nonsuppression on the dexamethasone suppression test, a blunted adrenocorticotropic hormone (ACTH) response to CRH, and hippocampal volume reduction. Evidence of HPA axis activation appears to have prognostic value and is associated with increased risk of depression relapse and even suicide.
Conclusion: Future research in this area will focus on a better understanding of the etiology and long-term consequences of HPA axis activation in depressed patients. In addition, medications that act on the HPA axis are currently in development and may be part of the psychiatrist's and primary care physician's pharmacopoeia in the near future.
Among psychiatric illnesses, major depressive disorder (MDD) is one of the most common, with a lifetime prevalence of greater than 17% in the general population.1 For many years, psychiatrists and neuroscientists have attempted to better understand the biology of MDD and other mood disorders. An extensive literature exists involving the role of neurotransmitters such as norepinephrine and serotonin in MDD. Hormone levels and the stress response have also been explored in MDD patients. As will be discussed, numerous investigators over the past 30 years have noted abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis in a subset of people with MDD. These findings led to enthusiasm that the use of so-called neuroendocrine challenges that enhance or attenuate HPA axis functioning might provide a “window to the brain” and, thus, a better understanding of the pathophysiology of MDD.2,3 Although the focus of the research and the techniques used to conduct the investigations have changed greatly over the past 4 decades, the role of the HPA axis in mood disorders remains an area of active research. In this review, we will discuss research regarding the HPA axis in people with depression, with an emphasis on how these findings are potentially important to clinical practice. In addition, the future of this field will be examined.
METHOD
The MEDLINE (1966–May 2001), PsychINFO (1887–March 2001), and Current Contents (1995–June 2001) databases were searched for pertinent articles on the HPA axis in patients with depression. In addition, hand searches were conducted on references from these sources and abstracts from meetings and books on this topic. Articles that would provide an overview of major or interesting studies in the field were selected for inclusion.
HPA Axis Regulation
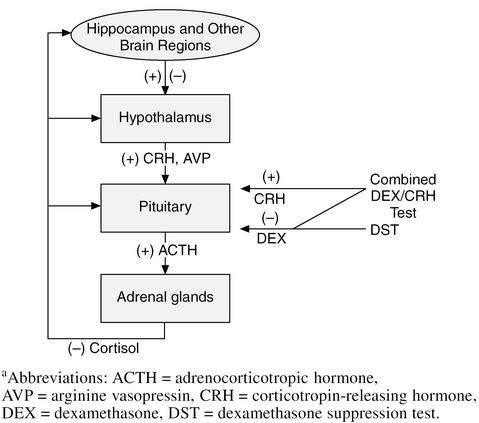
In this section, we will give a brief and somewhat simplified summary of the complex process of HPA axis regulation. The HPA axis, as the name implies, consists of a feedback loop including the hypothalamus, pituitary, and adrenal glands (Figure 1). In addition to these structures, the axis receives important regulation from the hippocampus, amygdala, bed nucleus of the stria terminalis (BNST), and paraventricular nuclei (PVN).
Figure 1.
The Hypothalamic-Pituitary-Adrenal Axisa
During a physical or emotional stressor, the HPA axis is activated. The hypothalamus secretes 2 hormones—corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP)—that act on the pituitary to increase adrenocorticotropin hormone (ACTH) release. CRH, also called corticotropin-releasing factor (CRF), is a 41–amino acid peptide distributed in various parts of the central nervous system and is the primary regulator of the mammalian stress response.4 CRH interacts with receptors on the pituitary that secrete ACTH (Figure 1). ACTH is carried in the blood to the adrenal cortex and interacts with receptors on adrenocortical cells that stimulate the production and release of cortisol. Cortisol is the adrenal glucocorticoid stress hormone secreted in humans and other primates. Some other animals, such as rats, produce a similar hormone called corticosterone. Most cortisol that circulates in the body is protein bound to corticosteroid-binding globulin. Only the unbound or “free” cortisol binds to receptors.
Cortisol binds to at least 2 types of receptors, both of which are located intracellularly. The first, called type I or mineralocorticoid receptors, has the highest affinity for cortisol and generally is almost completely bound by cortisol before the second, type II or glucocorticoid receptors, binds cortisol.5,6 These binding properties of cortisol differ from those of some synthetic corticosteroids, such as prednisone and dexamethasone (DEX), which bind fairly selectively to the type II receptor.
The loop is completed with the negative feedback of cortisol to the pituitary and hypothalamus. At least 2 forms of negative feedback to the HPA axis have been identified. The first is negative feedback of cortisol to the pituitary that is dependent on the concentration of cortisol. A second process termed fast feedback is dependent not on the absolute concentration of cortisol, but on the rate of change in concentration and involves interactions with receptors in the hypothalamus and hippocampus.7–10
HPA axis functioning is frequently examined using neuroendocrine challenge tests that attenuate or enhance cortisol, ACTH, or CRH release. The best known such test is the dexamethasone suppression test (DST), in which a dose of the synthetic corticosteroid DEX is given in the evening and cortisol samples are obtained the next day. The normal response is inhibition of cortisol release due to negative feedback by the DEX. If cortisol does not decrease below a certain level, then the patient is said to have “DST nonsuppression,” a finding that is frequently, but not always, associated with elevated cortisol levels. The DST is easily administered in outpatient settings both in depressed patients and in the diagnosis of Cushing's disease. A newer test that is increasingly used in research is the combined DEX/CRH challenge test in which the HPA axis is both stimulated by the administration of CRH and inhibited with DEX.11
HPA-Axis Findings in Depressed Patients
Both an excess of cortisol and DST nonsuppression have been reported for many years in patients with mood disorders.12,13 An analysis of more than 150 studies14 reported nonsuppression in 43% of persons with MDD, and in 67% with MDD with psychotic features (psychotic depression). Nonsuppression appears to be age dependent, increasing from only 34% in patients less than 18 years of age to 64% in patients more than 60 years of age. Studies have reported a positive correlation between DST nonsuppression and the number of depressive episodes.15,16 DST nonsuppression often returns to normal as the mood symptoms resolve. However, persistent DST nonsuppression is associated with a higher likelihood of MDD relapse than is DST normalization.17,18 Similar findings of increased relapse with persistent HPA axis dysregulation have recently been reported in depressed patients using the combined DEX/CRH challenge test.11,19–21 The combined DEX/CRH test shows a sensitivity up to 90% in detecting MDD.22
In part due to the observation that people with elevated cortisol caused by Cushing's disease frequently have mood symptoms, some investigators have suggested that elevated cortisol may be involved in the etiology of MDD.23,24 Another secretagogue that may be important in the pathophysiology of mood disorders is CRH. When CRH is administered to animals, it causes several of the symptoms of depression and anxiety disorders including reduced eating, decreased sexual behavior, disrupted sleep, alterations in locomotor activity, and abnormal response to novel stimuli.3,4 Additionally, when CRH is administered to primates, symptoms of depression, including decreased environmental exploration and increased huddling and lying down behaviors, are observed.25 Numerous studies have reported elevated cerebrospinal fluid concentrations of CRH in MDD patients.26–30 Increased CRH appears to be fairly specific to MDD, as normal levels are reported in schizophrenia, dementia,26 and mania.28 Elevated CRH appears to be a state rather than trait marker for depression, as these levels appear to normalize after the depression is treated.31
Another classic finding in MDD is a blunted ACTH response to CRH administration.3,11,32–36 This attenuated response may be secondary to a down-regulation of CRH receptors in the pituitary due to high levels of CRH during a depressive episode.3 However, some data suggest that lowering of cortisol levels with metyrapone normalizes the ACTH response to CRH.37 Thus, negative feedback by high circulating levels of cortisol may also contribute to blunted ACTH secretion. As with many other neuroendocrine findings with MDD, this blunted ACTH response to CRH appears to return to normal following resolution of depressive symptoms.38
HPA axis activation may be associated with structural changes in components of the axis. Possibly due to CRH hypersecretion, enlarged pituitary glands have been reported in 1 study of MDD patients,39 although another study reported no difference in volume from controls.40 Adrenal enlargement has also been observed in MDD patients.41,42 Once again, this appears to be a state rather than trait marker of MDD, as a significant decrease in volume after successful treatment is reported.42
Current Research Involving the HPA Axis in Mood Disorders
Current interest in the field focuses on 3 areas: (1) the long-term consequences on HPA axis abnormalities on the brain and other organ systems, (2) the role of early life experiences in the etiology of the HPA axis findings, and (3) the use of medications that act on the HPA axis in the treatment of depression.
The most extensive research on the effects of long-term elevations in cortisol in people with depression has focused on the brain. As the hippocampus is a brain region with a high concentration of glucocorticoid receptors, several studies have looked at hippocampal volume in people with MDD. Six studies have reported a significant reduction in hippocampal volume in MDD patients,43–48 while 2 studies have not.49,50 Although these findings are intriguing in light of extensive data suggesting changes in hippocampal structure and functioning following stress or corticosteroid exposure in animals, as we pointed out in a recent review,51 none of the above studies examined depressed patients with high cortisol and/or DST nonsuppression versus normal cortisol and/or DST suppression. Thus, one cannot determine if the smaller hippocampal volumes are due to cortisol excess. Some52–54 but not all55,56 studies also suggest greater cognitive impairment in patients with HPA axis abnormalities. As the hippocampus has important functions in memory performance, these data support the idea that cortisol may damage the hippocampus in depressed patients. However, an alternative explanation for all of the above findings is that of a small or dysfunctioning hippocampus prior to the onset of MDD, which may even predispose to the development of depression, as also discussed in our previous review.51 The idea of hippocampal differences predating the development of psychiatric illness has also been suggested to explain the atrophy seen in patients with posttraumatic stress disorder.57
Other organ systems also appear to be affected by cortisol elevation. Thakore et al.58 reported similar body mass index (BMI) but 2-fold greater intra-abdominal fat in MDD patients than controls.58 In this study, cortisol levels were significantly higher in the MDD patients than controls and correlated with the amount of intra-abdominal fat.58 Three studies have reported decreased bone density in people with MDD.59–61 Thus, MDD may be associated with some of the long-term consequences on physical health seen in people with Cushing's disease and after chronic corticosteroid therapy.
A second active area of research pertains to the etiology of HPA axis activation in MDD and other stress-related disorders. In animal models, a variety of environmental influences early in life are associated with later HPA axis changes (for a review see Heim et al.,62 Kaufman et al.63). Animal data suggest that stressful events, such as early maternal separation, are associated with increased CRH levels (a finding in people with depression) in adulthood.4,64,65 It is not known whether stressful experiences in youth lead to HPA axis changes and increased vulnerability in humans.
A third area of active research involves the development of medications that act on the HPA axis for the treatment of mood and anxiety disorders. Investigators have used medications already U.S. Food and Drug Administration–approved for other indications that inhibit enzymes involved in cortisol production (e.g., ketoconazole, metyrapone) in several small open-label studies with depressed patients with positive results (for a review see Brown et al.66). However, 2 double-blind placebo-controlled trials of ketoconazole in MDD have been published with mixed results.67,68 Medications that act as antagonists at the CRH or glucocorticoid receptor are also in development. One such medication is mifepristone, perhaps better known as RU 486. This medication induces abortions by antagonizing the progesterone. However, mifepristone is also a potent glucocorticoid receptor antagonist. Case reports and 1 double-blind placebo-controlled study suggest this medication may be useful for MDD.69,70 One study involving the use of a CRH antagonist in humans has been published.71 The authors reported that this medication was well tolerated and associated with reductions in depression and anxiety rating scale scores. Thus, it appears that in the future, medications that act on the HPA axis rather than catecholamines and serotonin may be in widespread use for the treatment of depression.
CONCLUSION
Abnormalities of the HPA axis are clearly common in patients with MDD. These findings may predict relapse of depression and be associated with adverse effects on the brain and other organs. Data suggesting structural changes in the brain and other organs due to elevated cortisol during depressive episodes are intriguing but inconclusive. Much future research will focus on interventions that modulate the HPA axis for the treatment of depression.
Drug names: dexamethasone (Decadron and others), ketoconazole (Nizoral and others).
Footnotes
Supported by grant MH-01725 from the National Institutes of Health, and the Theodore and Vada Stanley Foundation Bipolar Disorder Center at the University of Texas Southwestern Medical Center, Dallas (Dr. Brown), and the Summer Scholars Program, University of Texas Southwestern Medical Center, Dallas (Ms. Varghese).
Dr. Brown has received grant/research support from Corcept Therapeutics, Menlo Park, Calif.
REFERENCES
- Blazer DG, Kessler RC, McGonagle KA, et al. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- Pandey GN. Altered serotonin function in suicide: evidence from platelet and neuroendocrine studies. Ann N Y Acad Sci. 1997;836:182–200. doi: 10.1111/j.1749-6632.1997.tb52360.x. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci U S A. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Reul JMHM, Sutanto W. Corticosteroids and the brain. J Steroid Biochem Molec Biol. 1990;37:387–394. doi: 10.1016/0960-0760(90)90489-8. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Steroids, stability and stress. Front Neuroendocrinol. 1995;16:416–425. doi: 10.1006/frne.1995.1015. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Yates FE. Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann N Y Acad Sci. 1969;156:696–721. doi: 10.1111/j.1749-6632.1969.tb14008.x. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Jones MT. Effect of bilateral adrenalectomy and corticosteroid therapy on the secretion of corticotrophin-releasing factor activity from the hypothalamus of the rat in vitro. J Endocrinol. 1976;71:21–30. doi: 10.1677/joe.0.0710021. [DOI] [PubMed] [Google Scholar]
- Jones MT, Hillhouse EW. Structure-activity relationship and the mode of action of corticosteroid feedback on the secretion of corticotrophin-releasing factor (corticoliberin) J Steroid Biochem. 1976;7:1189–1202. doi: 10.1016/0022-4731(76)90054-6. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Murphy-Weinberg V, et al. Loss of glucocorticoid fast feedback in depression. Arch Gen Psychiatry. 1991;48:693–699. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben J, Wiedemann K, et al. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression: implications for pathophysiology of DST nonsuppression. Biol Psychiatry. 1987;22:228–234. doi: 10.1016/0006-3223(87)90237-x. [DOI] [PubMed] [Google Scholar]
- Sachar E. Cortisol production in depressive illness: a clinical and biochemical clarification. Arch Gen Psychiatry. 1971;23:289–298. doi: 10.1001/archpsyc.1970.01750040001001. [DOI] [PubMed] [Google Scholar]
- Carroll BJ. A specific laboratory test for the diagnosis of melancholia: standardization, validation, and clinical utility. J Clin Endocrinol Metab. 1981;51:433–437. doi: 10.1001/archpsyc.1981.01780260017001. [DOI] [PubMed] [Google Scholar]
- Arana GW, Ross JB, Ornsteen M. The dexamethasone suppression test for diagnosis and prognosis in psychiatry. Arch Gen Psychiatry. 1985;42:1193–1204. doi: 10.1001/archpsyc.1985.01790350067012. [DOI] [PubMed] [Google Scholar]
- Yerevanian BI, Privitera MR, Milanese E, et al. The dexamethasone suppression test during recurrent major depressive episodes. Biol Psychiatry. 1984;19:407–412. [PubMed] [Google Scholar]
- Lenox RH, Peyser JM, Rothschild B, et al. Failure to normalize the dexamethasone suppression test: association with length of illness. Biol Psychiatry. 1985;20:333–337. doi: 10.1016/0006-3223(85)90064-2. [DOI] [PubMed] [Google Scholar]
- Greden JF, Gardner R, King D, et al. Dexamethasone suppression tests in antidepressant treatment of melancholia. Arch Gen Psychiatry. 1983;40:493–500. doi: 10.1001/archpsyc.1983.01790050019002. [DOI] [PubMed] [Google Scholar]
- Targum SD. Persistent neuroendocrine dysregulation in major depressive disorder: a marker for early relapse. Biol Psychiatry. 1984;19:305–318. [PubMed] [Google Scholar]
- Holsboer-Trachsler E. Repeated administration of the combined dexamethasone human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatr Res. 1991;38:163–171. doi: 10.1016/0165-1781(91)90041-m. [DOI] [PubMed] [Google Scholar]
- Holsboer-Trachsler E, Hemmeter U, Hatzinger M, et al. Sleep deprivation and bright light as potential augmenters of antidepressant drug treatment: neurobiological and psychometric assessment of course. J Psychiatr Res. 1994;28:381–399. doi: 10.1016/0022-3956(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Schmider J, Lammers CH, Gotthardt V, et al. Combined dexamethasone/corticotropin-releasing hormone test in acute and remitted manic patients, in acute depression, and in normal controls, 1. Biol Psychiatry. 1995;38:797–802. doi: 10.1016/0006-3223(95)00064-X. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Murphy BEP. Steroids and depression. J Steroid Biochem Molec Biol. 1991;38:537–559. doi: 10.1016/0960-0760(91)90312-s. [DOI] [PubMed] [Google Scholar]
- Murphy BE, Wolkowitz OM. The pathophysiologic significance of hyperadrenocorticism: antiglucocorticoid strategies. Psychiatr Ann. 1993;23:682–690. [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, et al. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentration of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Arató M, Bánki CM, Nemeroff CB, et al. Hypothalamic-pituitary-adrenal axis and suicide. Ann N Y Acad Sci. 1986;487:263–270. doi: 10.1111/j.1749-6632.1986.tb27905.x. [DOI] [PubMed] [Google Scholar]
- Bánki CM, Bissette G, Arató M, et al. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144:873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- France RD, Urban B, Krishnan KRR, et al. CSF corticotropin-releasing factor-like immunoreactivity in chronic pain patients with and without major depression. Biol Psychiatry. 1988;23:86–88. doi: 10.1016/0006-3223(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Widerlöv E, Bissette G, Nemeroff CB. Monoamine metabolites, corticotropin-releasing factor and somatostatin as CSF markers in depressed patients. J Affect Disord. 1988;14:99–107. doi: 10.1016/0165-0327(88)90051-1. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Akil H, et al. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy: corticotropin-releasing factor, á–endorphin and somatostatin. Br J Psychiatry. 1991;158:59–63. doi: 10.1192/bjp.158.1.59. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Gerken A, von Bardeleben U, et al. Human corticotropin-releasing hormone in depression: correlation with thyrotropin secretion following thyrotropin-releasing hormone. Biol Psychiatry. 1986;21:601–611. doi: 10.1016/0006-3223(86)90121-6. [DOI] [PubMed] [Google Scholar]
- Gold PW, Loriaux DL, Roy A, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease: pathophysiologic and diagnostic implications. N Engl J Med. 1986;314:1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Maislin G, Winokur A, et al. Pituitary and adrenocortical responses to the ovine corticotropin-releasing hormone in depressed patients and healthy volunteers. Arch Gen Psychiatry. 1987;44:775–781. doi: 10.1001/archpsyc.1987.01800210019003. [DOI] [PubMed] [Google Scholar]
- Kathol RG, Jaeckle RS, Lopez JF, et al. Consistent reduction of ACTH responses to stimulation with CRH, vasopressin and hypoglycaemia in patients with major depression. Br J Psychiatry. 1989;155:468–478. doi: 10.1192/bjp.155.4.468. [DOI] [PubMed] [Google Scholar]
- Young EA, Watson SJ, Kotun J, et al. β-lipotropin-β-endorphin response to low-dose ovine corticotropin-releasing factor in endogenous depression. Arch Gen Psychiatry. 1990;47:449–457. doi: 10.1001/archpsyc.1990.01810170049008. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Stalla GK, Müller OA, et al. Blunting of ACTH response to human CRH in depressed patients is avoided by metyrapone pretreatment. Biol Psychiatry. 1988;24:782–786. doi: 10.1016/0006-3223(88)90254-5. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Maislin G, Winokur A, et al. The oCRH stimulation test before and after clinical recovery from depression. J Affect Disord. 1988;14:213–222. doi: 10.1016/0165-0327(88)90037-7. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Doraiswamy PM, Lurie SN, et al. Pituitary size in depression. J Clin Endocrinol Metab. 1991;72:256–259. doi: 10.1210/jcem-72-2-256. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Nicoletti M, Brambilla P, and et al. MR volumetric analysis of pituitary gland in bipolar and unipolar disorder patients. [poster abstract 104]. Presented at the 4th International Conference on Bipolar Disorder; June 14–16, 2001; Pittsburgh, Pa. [Google Scholar]
- Nemeroff CB, Krishnan KR, Reed D, et al. Adrenal gland enlargement in major depression: a computed tomographic study. Arch Gen Psychiatry. 1992;49:384–387. doi: 10.1001/archpsyc.1992.01820050048008. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Phillips JJ, Sadow TF, et al. Adrenal gland volume in major depression: increase during the depressive episode and decrease with successful treatment. Arch Gen Psychiatry. 1995;52:213–218. doi: 10.1001/archpsyc.1995.03950150045009. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, et al. Cortical grey matter reduction associated with treatment: resistant chronic unipolar depression. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Soc Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, et al. Quantitative MRI of the hippocampal and amygdala in severe depression. Psychol Med. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Doraiswamy PM, McDonald WM, et al. Hypercortisolemia and hippocampal changes in depression. Psychiatr Res. 1993;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Fox NC, Cipolotti L, et al. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci. 2000;12:493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology. 1999;21:474–484. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Post RM, Savard R, et al. Cortisol hypersecretion and cognitive impairment in depression. Arch Gen Psychiatry. 1984;41:279–283. doi: 10.1001/archpsyc.1984.01790140069008. [DOI] [PubMed] [Google Scholar]
- Winokur G, Black DW, Nasrallah A. DST nonsuppressor status: relationship to specific aspects of the depressive syndrome. Biol Psychiatry. 1987;22:360–368. doi: 10.1016/0006-3223(87)90153-3. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry. 1990;147:1297–1303. doi: 10.1176/ajp.147.10.1297. [DOI] [PubMed] [Google Scholar]
- Georgotas A, McCue RE, Kim M, et al. Dexamethasone suppression in dementia, depression, and normal aging. Am J Psychiatry. 1986;143:452–456. doi: 10.1176/ajp.143.4.452. [DOI] [PubMed] [Google Scholar]
- Caine ED, Yerevanian BI, Bamford KA. Cognitive function and the dexamethasone suppression test in depression. Am J Psychiatry. 1984;141:116–118. doi: 10.1176/ajp.141.1.116. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Hippocampal diminution in PTSD: more (or less?) than meets the eye. Hippocampus. 2001;11:73–74. doi: 10.1002/hipo.1022. [DOI] [PubMed] [Google Scholar]
- Thakore JH, Richards PJ, Reznek RH, et al. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry. 1997;41:1140–1142. doi: 10.1016/S0006-3223(97)85394-2. [DOI] [PubMed] [Google Scholar]
- Michelson D, Stratakis C, Hill L, et al. Bone mineral density in women with depression. N Engl J Med. 1996;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- Schweiger U, Deuschle M, Korner A, et al. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry. 1994;151:1691–1693. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- Schweiger U, Weber B, Deuschle M, et al. Lumbar bone mineral density in patients with major depression: evidence of increased bone loss at follow-up. Am J Psychiatry. 2000;157:118–120. doi: 10.1176/ajp.157.1.118. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, et al. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder: focus on corticotropin-releasing factor. Ann N Y Acad Sci. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, et al. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Brown ES, Bobadilla L, Rush AJ. Ketoconazole in bipolar patients with depressive symptoms: a case series and literature review. Bipolar Disord. 2001;3:23–29. doi: 10.1034/j.1399-5618.2001.030103.x. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Chan T, et al. Antiglucocorticoid treatment of depression: double-blind ketoconazole. Biol Psychiatry. 1999;45:1070–1074. doi: 10.1016/s0006-3223(98)00267-4. [DOI] [PubMed] [Google Scholar]
- Malison RT, Anand A, Pelton GH, et al. Limited efficacy of ketoconazole in treatment-refractory major depression. J Clin Psychopharmacol. 1999;19:466–470. doi: 10.1097/00004714-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Murphy BE, Filipini D, Ghadirian AM. Possible use of glucocorticoid receptor antagonists in the treatment of major depression: preliminary results using RU 486. J Psychiatry Neurosci. 1993;18:209–213. [PMC free article] [PubMed] [Google Scholar]
- Belanoff JK, Flores BH, Kalezhan M, and et al. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. In press. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]