Abstract
FR264205 is a novel parenteral 3′-aminopyrazolium cephalosporin. This study evaluated the in vitro and in vivo activities of FR264205 against Pseudomonas aeruginosa. The MIC of FR264205 at which 90% of 193 clinical isolates of P. aeruginosa were inhibited was 1 μg/ml, 8- to 16-fold lower than those of ceftazidime (CAZ), imipenem (IPM), and ciprofloxacin (CIP). FR264205 also exhibited this level of activity against CAZ-, IPM-, and CIP-resistant P. aeruginosa. The reduction in the susceptibility of FR264205 by AmpC β-lactamase was lower than that of CAZ, indicating a relatively high stability of FR264205 against AmpC β-lactamase, the main resistance mechanism for cephalosporins. Neither expression of efflux pumps nor deficiency of OprD decreased the activity of FR264205. No spontaneous resistance mutants were selected in the presence of FR264205, and the reduction in susceptibility to FR264205 was lower than that to CAZ, IPM, and CIP after serial passage, suggesting that FR264205 has a low propensity for selecting resistance. In murine pulmonary, urinary tract, and burn wound models of infection caused by P. aeruginosa, the efficacy of FR264205 was superior or comparable to those of CAZ and IPM. These results indicate that FR264205 should have good potential as an antibacterial agent for P. aeruginosa.
Nosocomial infections with gram-negative bacteria are a major problem for immunocompromised patients. Pseudomonas aeruginosa exhibits considerable inherent resistance, caused by low outer membrane permeability, multiple efflux pumps, and chromosomal AmpC β-lactamase (12). P. aeruginosa can also acquire additional resistance mechanisms, such as constitutive production of AmpC β-lactamase, OprD loss, and overproduction of efflux pumps. Although ceftazidime (CAZ) has been used as a first-line drug for P. aeruginosa infection, resistant mutants showing constitutive AmpC β-lactamase production can be selected in clinical settings, leading to therapeutic failure (2). P. aeruginosa has developed resistance not only to cephalosporins but also to carbapenems and quinolones. In 2003, the National Nosocomial Infections Surveillance System reported that resistance rates of P. aeruginosa to imipenem, quinolone, and broad-spectrum cephalosporins were 21.1, 29.5, and 31.9%, respectively. Compared to rates in the period between 1998 and 2002, these rates were increased by 15, 9, and 20%, respectively (15). Therefore, there is a critical need for new anti-P. aeruginosa agents that have no cross-resistance to currently marketed antibacterial agents and low propensities for inducing resistance.
In order to generate promising anti-P. aeruginosa agents, research by our group has been directed toward the development of novel cephalosporins. As a result of exploration of structure-activity relationships of 3-(2,4-disubstituted 3-aminopyrazolio)methyl cephalosporins, FR264205 was discovered (Fig. 1). The antibacterial spectrum of FR264205 was similar to that of CAZ, and the MICs of FR264205 for Staphylococcus aureus ATCC 29213, Streptococcus pneumoniae ATCC 6305, Klebsiella pneumoniae IFO3512, and Haemophilus influenzae ATCC 9334 were 32, 0.25, 0.0625, and 0.25 μg/ml, respectively. In this study, the in vitro antibacterial characteristics of FR264205 against P. aeruginosa were compared with those of CAZ, imipenem (IPM), and ciprofloxacin (CIP). In addition, the efficacy of FR264205 against pulmonary, urinary tract, and burn wound infections caused by P. aeruginosa was evaluated in mice.
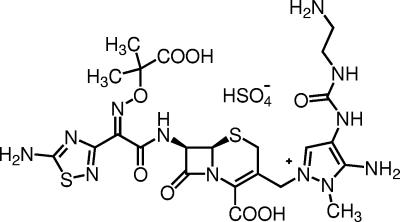
FIG. 1.
Chemical structure of FR264205.
(This work was presented in part at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2005 [6].)
MATERIALS AND METHODS
Compounds.
FR264205 was synthesized at Astellas Pharma, Inc., formerly Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan. CAZ, IPM, and CIP were purchased from Japan GlaxoSmithKline K. K. (Tokyo, Japan), Banyu Pharmaceutical Co., Ltd. (Tokyo, Japan), and Bayer, Ltd. (Osaka, Japan), respectively. All drugs were prepared just before use and were used as pure free-acid equivalents.
Organisms.
A total of 193 clinical isolates of P. aeruginosa were isolated from clinical specimens in 2002 in Japan. The criteria for drug resistance were MICs of ≥32 μg/ml CAZ, ≥16 μg/ml IPM, and ≥4 μg/ml CIP for resistant P. aeruginosa strains.
To determine the effects of classical β-lactamases and extended-spectrum β-lactamases (ESBLs), Escherichia coli C600 was used as a host strain. TEM-1, TEM-2, OXA-2, CTX-M-3, and CTX-M-18 were introduced by conjugation with clinical isolates. SHV-1 was introduced by transformation of a plasmid extracted from a clinical isolate. OXA-1 was introduced by transformation with a plasmid containing a cloned copy of the β-lactamase gene. E. coli C600 producing TEM-3, TEM-4, TEM-5, TEM-6, TEM-7, TEM-8, TEM-9, SHV-2, SHV-3, and SHV-4 and plasmid-cloned OXA-1 were kindly provided by G. A. Jacoby, Massachusetts General Hospital, Boston, MA (7, 11). Clinical isolates producing TEM-1, TEM-2, SHV-1, OXA-2, CTX-M-3, and CTX-M-18 were kindly provided by Y. Ishii, Toho University School of Medicine, Tokyo, Japan. To determine the effect of AmpC β-lactamase and metallo-β-lactamases, the following P. aeruginosa strains were used: FP1380, a clinical isolate constitutively producing AmpC β-lactamase; PAO4069, a parent strain that inducibly produces AmpC β-lactamase; PAO1456, a constitutively AmpC β-lactamase-producing spontaneous mutant of PAO4069; and 22029, a clinical isolate producing IMP-1 metallo-β-lactamase. P. aeruginosa PAO4069 and PAO1456 were kindly provided by H. Matsumoto, Shinshu University School of Medicine, Matsumoto, Japan. To determine the effect of efflux pumps, the following isogenic sets of P. aeruginosa strains were used: MexAB-OprM-expressing P. aeruginosa KG2212, containing a disrupted mexR regulator gene, derived from PAO1 (5); MexCD-OprJ-expressing P. aeruginosa KG3056, formerly CDR6, a spontaneous mutant derived from PAO selected on Mueller-Hinton (MH) II agar containing cefpirome and ofloxacin (13); MexEF-OprN-expressing P. aeruginosa KG4001, containing a spontaneous mutation of an nfxC regulator gene, derived from PAO4222 (10); and MexXY-expressing P. aeruginosa KG4545, containing a disrupted mexZ regulator gene, derived from PAO1 (N. Gotoh, unpublished data). To determine the effect of OprD, P. aeruginosa KG5013, containing a disrupted oprD gene, derived from PAO1, was used (N. Gotoh, unpublished data). P. aeruginosa KG2212, KG3056, KG4001, KG4545, KG5013, PAO1, and PAO4222 were kindly provided by N. Gotoh, Kyoto Pharmaceutical University, Kyoto, Japan. To determine the in vivo efficacy, P. aeruginosa 93, a clinical isolate from Japan, was used.
MIC determination.
MICs were determined by the standard agar dilution method according to reference method M7-A7 of the National Committee for Clinical Laboratory Standards, now known as the Clinical and Laboratory Standards Institute, with MH agar (Becton Dickinson, Tokyo, Japan) (14). An inoculum of 104 CFU per spot was inoculated onto agar plates containing twofold serial dilutions of antibacterial agents. After aerobic incubation for 18 to 20 h at 35°C, the MIC was defined as the lowest drug concentration that prevented visible growth.
Selection of spontaneous mutants.
Spontaneous mutants were selected by plating duplicate overnight broth cultures of P. aeruginosa PAO1 onto MH agar containing antibacterial agents at doubling concentrations to 4× to 16× MIC. After incubation for 48 h, colonies that grew on the plate were counted. Mutation frequencies were calculated, with results of triplicate plate counts on antibiotic-free agar used as the denominator.
Development of resistance by serial passage.
Serial passage of P. aeruginosa PAO1 was performed daily using freshly prepared MH broth containing a series of twofold dilutions of antibacterial agents. For each subsequent daily passage, the inoculum was taken from the tube which had a concentration of one-fourth the MIC. Strains were passaged for five consecutive days.
In vivo efficacy against experimental infection.
ICR mice (4 weeks of age) were purchased from Japan SLC, Inc. (Hamamatsu, Japan), housed in cages, and given food and water ad libitum in animal rooms maintained at 23 ± 2°C with 55% ± 20% relative humidity. On the day of infection, the mice were anesthetized intravenously with pentobarbital at 50 mg/kg of body weight. On the day after the last treatment, the mice were sacrificed by CO2 asphyxiation. Six mice were used for each dose. All animal experimental procedures complied with the guidelines of the Animal Experiment Committee, Astellas Pharma, Inc. To prepare inoculum, P. aeruginosa 93 was grown on tryptic soy agar (Eiken Chemical Co., Ltd., Tokyo, Japan) at 35°C for 20 h and then suspended in saline.
(i) Pulmonary infection.
Neutropenia was induced in male mice by intraperitoneal administration of 200 mg/kg cyclophosphamide 4 days before infection. Anesthetized mice were challenged intranasally with 3.30 log10 CFU of P. aeruginosa 93. FR264205, CAZ, and IPM at doses of 2 and 10 mg/kg were administered by subcutaneous injection twice daily for 3 days, starting at 3 h after infection. Lungs from sacrificed mice were removed aseptically, and viable bacterial counts were determined.
(ii) Urinary tract infection.
Female mice were denied water for 1 day before bacterial challenge. Anesthetized mice were challenged via the uterine opening with 4.32 log10 CFU of P. aeruginosa 93, and the uterine opening was then clamped for 5 h to prevent urine flow. FR264205, CAZ, and IPM at doses of 0.5 and 2 mg/kg were administered by subcutaneous injection at 5 h postchallenge plus twice daily for 2 days. Bilateral kidneys from sacrificed mice were removed aseptically, and viable bacterial counts were determined.
(iii) Burn wound infection.
Male anesthetized mice were given an ethanol flame burn injury (0.1 ml of flaming ethanol was applied twice to their shaved backs) and then challenged with 4.60 log10 CFU of P. aeruginosa 93 at the site of the burn wound. FR264205, CAZ, and IPM at doses of 10 and 50 mg/kg were administered by intravenous injection twice daily for 3 days, starting at 3 h after infection. Burn wound lesions from sacrificed mice were removed aseptically, and viable bacterial counts were determined.
Statistical analysis.
Tukey's multiple-comparison test was performed to determine statistically significant differences between treatment groups at the same doses. A P value of <0.05 was considered significant.
RESULTS
Antibacterial activity against clinical isolates of P. aeruginosa.
In Table 1, the MIC of FR264205 is compared to those of CAZ, IPM, and CIP for clinical isolates of P. aeruginosa. Against 193 isolates of P. aeruginosa, the MIC of FR264205 at which 90% of isolates were inhibited (MIC90) was 1 μg/ml, 8- to 16-fold more potent than other agents. Also, the MIC range of FR264205 was 0.25 to 4 μg/ml, the narrowest range of agents tested. FR264205 exhibited this level of activity against CAZ-, IPM-, and CIP-resistant P. aeruginosa strains.
TABLE 1.
Antibacterial activity of FR264205 against clinical isolates of P. aeruginosa
| Strain (no. of isolates) | Compound | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| P. aeruginosa (193) | FR264205 | 0.25-4 | 0.5 | 1 |
| CAZ | 0.125-128 | 2 | 16 | |
| IPM | 0.125-64 | 2 | 16 | |
| CIP | ≤0.0313->128 | 0.25 | 8 | |
| P. aeruginosa, CAZ | FR264205 | 1-4 | 2 | 4 |
| resistant (13) | CAZ | 32-128 | 64 | 128 |
| IPM | 1-32 | 16 | 32 | |
| CIP | 0.0625-64 | 2 | 64 | |
| P. aeruginosa, IPM | FR264205 | 0.5-1 | 0.5 | 1 |
| resistant, CAZ | CAZ | 1-16 | 4 | 16 |
| susceptible (35) | IPM | 16-64 | 16 | 32 |
| CIP | 0.0625-32 | 1 | 8 | |
| P. aeruginosa, CIP | FR264205 | 0.5-4 | 1 | 2 |
| resistant (30) | CAZ | 1-128 | 8 | 64 |
| IPM | 1-32 | 8 | 16 | |
| CIP | 4->128 | 8 | 64 | |
Antibacterial activity against strains producing β-lactamases.
Table 2 shows the MICs of FR264205 for strains producing various β-lactamases. Classical β-lactamases, such as TEM-1, TEM-2, SHV-1, and OXA-1, had minimal effects on the MIC of FR264205. ESBLs reduced the activity of FR264205, but FR264205 showed higher activity than CAZ against strains producing TEM-3, TEM-4, TEM-8, SHV-2, SHV-3, and SHV-4. ESBLs had no effect on the MIC of IPM, as expected. Against P. aeruginosa-producing metallo-β-lactamase, FR264205 had no activity, similar to CAZ and IPM. Against P. aeruginosa FP1380 constitutively producing AmpC β-lactamase, FR264205 showed >32-fold-higher activity than CAZ. The MIC of FR264205 for P. aeruginosa PAO1456, constitutively producing AmpC β-lactamase, was twofold higher than it was for PAO4069, the parent strain, while the MIC of CAZ was16-fold higher, suggesting a relatively high stability of FR264205 against AmpC β-lactamase.
TABLE 2.
Antibacterial activity of FR264205 against strains producing β-lactamases
| Strain and β-lactamase produced | β-Lactamase | MIC (μg/ml)
|
||
|---|---|---|---|---|
| FR264205 | CAZ | IPM | ||
| E. coli C600 | ||||
| TEM-1 | Group 2ba | 0.25 | 0.25 | 0.125 |
| TEM-2 | Group 2ba | 0.5 | 1 | 0.25 |
| SHV-1 | Group 2ba | 0.5 | 1 | 0.25 |
| OXA-1 | Group 2da | 0.5 | 0.5 | 0.25 |
| TEM-3 | ESBL | 1 | 32 | 0.25 |
| TEM-4 | ESBL | 2 | 32 | 0.25 |
| TEM-5 | ESBL | 32 | 32 | 0.25 |
| TEM-6 | ESBL | 32 | 64 | 0.25 |
| TEM-7 | ESBL | 32 | 64 | 0.25 |
| TEM-8 | ESBL | 16 | 128 | 0.25 |
| TEM-9 | ESBL | 32 | 32 | 0.25 |
| SHV-2 | ESBL | 32 | 128 | 0.25 |
| SHV-3 | ESBL | 32 | >128 | 0.25 |
| SHV-4 | ESBL | 16 | 128 | 0.25 |
| OXA-2 | ESBL | 4 | 4 | 0.25 |
| CTX-M-3 | ESBL | 16 | 4 | 0.25 |
| CTX-M-18 | ESBL | 16 | 4 | 0.25 |
| E. coli C600 host | 0.25 | 0.25 | 0.25 | |
| P. aeruginosa | ||||
| 22029 | Metallo | >128 | >128 | 128 |
| FP1380 | Constitutive | 4 | >128 | 0.5 |
| AmpC | ||||
| PAO1456 (spontaneous | Constitutive | 1 | 32 | 1 |
| mutant of PAO4069) | AmpC | |||
| PAO4069 | Inducible | 0.5 | 2 | 1 |
| AmpC | ||||
Bush-Jacoby-Medeiros group (1).
Antibacterial activity against P. aeruginosa-producing efflux pumps and P. aeruginosa lacking OprD.
Table 3 shows the MIC of FR264205 for P. aeruginosa, which overexpresses MexAB-OprM, MexCD-OprJ, MexEF-OprN, or MexXY. Expression of efflux pumps had no effect on the MIC of FR264205, while MexCD-OprJ and MexEF-OprN increased the MIC of CIP 16-fold.
TABLE 3.
Antibacterial activity of FR264205 against P. aeruginosa isolates producing efflux pumps
| Strain | Efflux pump | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| FR264205 | CAZ | IPM | CIP | ||
| KG2212 | MexAB-OprM | 0.5 | 2 | 1 | 0.25 |
| KG3056 | MexCD-OprJ | 0.25 | 0.5 | 0.5 | 2 |
| KG4545 | MexXY | 0.5 | 2 | 1 | 0.25 |
| PAO1 | Parent | 0.5 | 2 | 1 | 0.125 |
| KG4001a | MexEF-OprN | 0.5 | 2 | 4 | 2 |
| PAO4222 | Parent | 0.5 | 2 | 1 | 0.125 |
The expression of OprD is decreased.
The MICs of FR264205 and IPM for P. aeruginosa KG5013, an OprD− mutant of P. aeruginosa PAO1, were 0.5 and 16 μg/ml, respectively. Compared with the effect on the MICs for P. aeruginosa PAO1, OprD loss had no effect on the MIC of FR264205 but increased the MIC of IPM 16-fold.
Frequency of spontaneous resistance.
Table 4 shows the frequency of spontaneous resistance of P. aeruginosa PAO1. No colonies were selected on agar plates containing FR264205. The frequency of resistance to FR264205 was lower than that to CAZ at every tested concentration and lower than those to IPM and CIP at 4× MIC.
TABLE 4.
Frequencies of spontaneous mutations in P. aeruginosa PAO1
| Concn | Mutation frequency (10−9)
|
|||
|---|---|---|---|---|
| FR264205 | CAZ | IPM | CIP | |
| 4× MIC | <6.1 | 430 | >1,000 | 34 |
| 8× MIC | <6.1 | 370 | <6.1 | <6.1 |
| 16× MIC | <6.1 | 120 | <6.1 | <6.1 |
Development of resistance by serial passage.
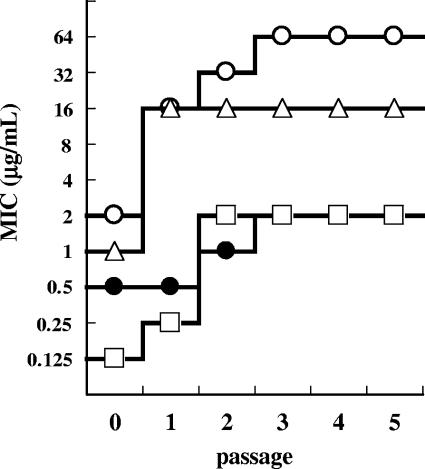
Figure 2 shows the reduction in the susceptibility of P. aeruginosa PAO1 to FR264205 from serial passage. After five serial passages, the reduction in susceptibility to FR264205 was at its lowest (fourfold reduction), and the final MIC was 2 μg/ml. In contrast, the susceptibilities of CAZ, IPM, and CIP were reduced 32-, 16-, and 16-fold, respectively. A significant reduction in susceptibility was seen after a single passage of the strain in dilutions of CAZ and IPM.
FIG. 2.
Development of resistance in P. aeruginosa PAO1 after serial passage. P. aeruginosa PAO1 was cultured in medium containing FR264205 (•), CAZ (○), IPM (▵), and CIP (□).
Efficacy against pulmonary infection.
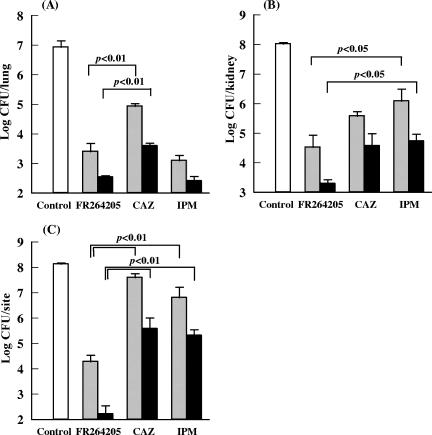
The MICs of FR264205, CAZ, and IPM for P. aeruginosa 93 were 0.25, 1, and 1 μg/ml, respectively. After infection with the strain at 3.30 log10 CFU, the bacterial count in the lungs of untreated control mice increased to 6.93 ± 0.22 log10 CFU. As shown in Fig. 3A, FR264205 (2 and 10 mg/kg) was highly effective, and the efficacy of FR264205 was comparable to that of IPM and significantly better than that of CAZ (P < 0.01).
FIG. 3.
Therapeutic activity of FR264205 in pulmonary infection (A), urinary tract infection (B), and burn wound infection (C) caused by P. aeruginosa 93. Gray and filled columns indicate 2-mg/kg and 10-mg/kg doses, respectively (A), 0.5-mg/kg and 2-mg/kg doses, respectively (B), and 10-mg/kg and 50-mg/kg doses, respectively (C).
Efficacy against urinary tract infection.
Figure 3B shows the efficacy of FR264205 in a urinary tract infection model. FR264205 exhibited efficacy at doses from 0.5 mg/kg, and the efficacy was significantly better than that of IPM (P < 0.05). The reduction in the bacterial count was more marked following treatment with FR264205 than with CAZ, although the difference was not significant.
Efficacy against burn wound infection.
Figure 3C shows the efficacy of FR264205 in a burn wound infection model. The efficacy of FR264205 (10 and 50 mg/kg) was significantly better than those of CAZ and IPM (P < 0.01). FR264205 at a dose of 50 mg/kg reduced the bacterial count by approximately 6 log CFU/g per lesion compared with that for untreated controls.
DISCUSSION
In this study, the antibacterial activity against P. aeruginosa of FR264205, a new cephalosporin, was evaluated. Results indicate that FR264205 possesses superior activity with a markedly lower MIC90 than CAZ, IPM, and CIP. It was also noteworthy that FR264205 was strongly effective against P. aeruginosa strains resistant to CAZ, IPM, and CIP.
The main resistance mechanisms for P. aeruginosa are constitutive AmpC β-lactamase overproduction, efflux pumps, loss of OprD, ESBLs, and metallo β-lactamase (8, 12); therefore, the effect of these mechanisms on the antibacterial activity of FR264205 was analyzed. The MIC of FR264205 against P. aeruginosa constitutively producing AmpC β-lactamase was more potent than that of CAZ. Also, the reduction in the susceptibility of FR264205 by overproduction of AmpC β-lactamase was lower than for CAZ, suggesting a relatively high stability of FR264205 against AmpC β-lactamase. Efflux pumps and OprD had no effect on the MIC of FR264205, as expected, as these are the main resistance mechanisms against quinolones and carbapenems, respectively. These results provide evidence that FR264205 shows no cross-resistance to CAZ, IPM, and CIP. On the other hand, ESBLs and a metallo-β-lactamase reduced the activity of FR264205. Considering the MIC90 values of FR264205, the clinical isolates of P. aeruginosa used in this study probably did not include strains producing ESBLs or metallo-β-lactamases.
In addition to activity against strains with known resistance mechanisms, the incidence of mutants resistant to FR264205 was analyzed in two experiments testing the frequency of spontaneous resistance and the development of resistance by serial passage. Both experiments showed that FR264205 has a lower propensity for inducing resistance than CAZ, IPM, and CIP. With FR264205, it is difficult to select resistant mutants, perhaps due to its high stability against AmpC β-lactamase. Recent clinical failures in treatments for P. aeruginosa infections have been caused mainly by resistance developing during treatment (9), so we can expect a lower possibility of clinical failures during FR264205 treatment.
P. aeruginosa is a frequent clinical cause of pulmonary, urinary tract, and burn wound infections; therefore, the in vivo therapeutic efficacy of FR264205 was assessed in murine infection models (1a, 3, 4, 16). The efficacy of FR264205 was superior or comparable to those of CAZ and IPM, with a marked reduction in bacterial count. Also, the efficacy of FR264205 against a clinical isolate of P. aeruginosa resistant to CAZ and IPM was determined. As expected from the lack of cross-resistance of FR264205 with CAZ and IPM in vitro, FR264205 was effective in all infection models, with the efficacy of FR264205 superior to that of CAZ and IPM (unpublished results). The pharmacokinetics of FR264205 were similar to those of CAZ in animals (unpublished results), and we assume that human pharmacokinetics will be similar. If so, FR264205 should exhibit therapeutic effects at clinically available doses.
In conclusion, FR264205 has potent in vitro and in vivo antibacterial activities against P. aeruginosa, with no cross-resistance to currently marketed antibacterial agents and a lower propensity for inducing resistance. FR264205 is a promising compound suitable for further evaluation as a new antibacterial cephalosporin candidate against P. aeruginosa.
Acknowledgments
We are grateful to David Barrett, Chemistry Research Laboratories, Astellas Pharma, Inc., for kind help and advice.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Campana, S., G. Taccetti, N. Ravenni, I. Masi, S. Audino, B. Sisi, T. Repetto, G. Doring, and M. de Martino. 2004. Molecular epidemiology of Pseudomonas aeruginosa, Burkholderia cepacia complex and methicillin-resistant Staphylococcus aureus in a cystic fibrosis center. J. Cyst. Fibros. 3:159-163. [DOI] [PubMed] [Google Scholar]
- 2.De Champs, C., L. Poirel, R. Bonnet, D. Sirot, C. Chanal, J. Sirot, and P. Nordmann. 2002. Prospective survey of β-lactamases produced by ceftazidime-resistant Pseudomonas aeruginosa isolated in a French hospital in 2000. Antimicrob. Agents Chemother. 46:3031-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geyik, M. F., M. Aldemir, S. Hosoglu, and H. I. Tacyildiz. 2003. Epidemiology of burn unit infections in children. Am. J. Infect. Control 31:342-346. [DOI] [PubMed] [Google Scholar]
- 4.Gordon, K. A., R. N. Jones, and SENTRY Participant Groups (Europe, Latin America, North America). 2003. Susceptibility patterns of orally administered antimicrobials among urinary tract infection pathogens from hospitalized patients in North America: comparison report to Europe and Latin America. Results from the SENTRY Antimicrobial Surveillance Program (2000). Diagn. Microbiol. Infect. Dis. 45:295-301. [DOI] [PubMed] [Google Scholar]
- 5.Gotoh, N., H. Tsujimoto, M. Tsuda, K. Okamoto, A. Nomura, T. Wada, M. Nakahashi, and T. Nishino. 1998. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:1938-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatano, K., S. Takeda, T. Nakai, F. Ikeda, K. Maki, K. Matsuda, F. Matsumoto, and S. Kuwahara. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1452.
- 7.Jacoby, G. A., and L. Sutton. 1991. Properties of plasmids responsible for production of extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 35:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, R. N., L. M. Deshpande, J. M. Bell, J. D. Turnidge, S. Kohno, Y. Hirakata, Y. Ono, Y. Miyazawa, S. Kawakama, M. Inoue, Y. Hirata, and M. A. Toleman. 2004. Evaluation of the contemporary occurrence rates of metallo-β-lactamases in multidrug-resistant Gram-negative bacilli in Japan: report from the SENTRY Antimicrobial Surveillance Program (1998-2002). Diagn. Microbiol. Infect. Dis. 49:289-294. [DOI] [PubMed] [Google Scholar]
- 9.Juan, C., O. Gutierrez, A. Oliver, J. I. Ayestaran, N. Borrell, and J. L. Perez. 2005. Contribution of clonal dissemination and selection of mutants during therapy to Pseudomonas aeruginosa antimicrobial resistance in an intensive care unit setting. Clin. Microbiol. Infect. 11:887-892. [DOI] [PubMed] [Google Scholar]
- 10.Kohler, T., M. Michea-Hamzehpour, U. Henze, N. Gotoh, L. K. Curty, and J. C. Pechere. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:345-354. [DOI] [PubMed] [Google Scholar]
- 11.Levesque, R. C., A. A. Medeiros, and G. A. Jacoby. 1987. Molecular cloning and DNA homology of plasmid-mediated β-lactamase genes. Mol. Gen. Genet. 206:252-258. [DOI] [PubMed] [Google Scholar]
- 12.Livermore, D. M. 2001. Of Pseudomonas, porins, pumps and carbapenems. J. Antimicrob. Chemother. 47:247-250. [DOI] [PubMed] [Google Scholar]
- 13.Masuda, N., N. Gotoh, S. Ohya, and T. Nishino. 1996. Quantitative correlation between susceptibility and OprJ production in NfxB mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 40:909-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 16.Santucci, S. G., S. Gobara, C. R. Santos, C. Fontana, and A. S. Levin. 2003. Infections in a burn intensive care unit: experience of seven years. J. Hosp. Infect. 53:6-13. [DOI] [PubMed] [Google Scholar]