Abstract
Mass spectrometry (MS) represents a rapid technique for the identification of microbial monocultures, and its adaptation to the detection of pathogens in real-world samples is a public health and homeland security priority. Norovirus, a leading cause of gastroenteritis in the world, is difficult to monitor because it cannot be cultured outside the human body. The detection of norovirus capsid protein was explored using three common MS-based methods: scanning of intact proteins, peptide mass fingerprinting, and peptide sequencing. Detection of intact target protein was limited by poor selectivity and sensitivity. Detection of up to 16 target peptides by peptide mass fingerprinting allowed for the reproducible and confident (P < 0.05) detection of the 56-kDa norovirus capsid protein in the range of 0.1 × 10−12 to 50 × 10−12 mol in authentic standards of recombinant norovirus virus-like particles (VLPs). To explore assay performance in complex matrixes, a non-gel-based, rapid method (2 to 3 h) for virus extraction from human stool was evaluated (72% ± 12% recovery), and additional analyses were performed on norovirus-free stool extracts fortified with VLPs. Whereas peptide mass fingerprinting was rendered impractical by sample interferences, peptide sequencing using nanospray tandem MS facilitated unambiguous identification of ≥250 fmol of capsid protein in stool extracts. This is the first report on MS-based detection of norovirus, accomplished by using structurally identical, noninfective VLPs at clinically relevant concentrations. It represents an important milestone in the development of assays for surveillance of this category B bioterrorism agent.
Noroviruses, formerly known as Norwalk-like viruses and small round-structured viruses in the family Caliciviridae, are human pathogens responsible for outbreaks of waterborne, food-borne, person-to-person, hospital, and recreational-water-contact origin (3, 5, 13, 27). Estimated to cause more than 23 million cases of gastroenteritis per year in the United States, norovirus infections are second only to the common cold in disease occurrence (32). The actual number of norovirus cases is unknown, since the disease is not reportable and is difficult to diagnose. Between 1991 and 2000, there was an 11-fold increase in reports of confirmed outbreaks of norovirus (33). In the past 5 years, infections have remained pervasive (32), making virus surveillance a top priority. An added incentive for the development of rapid detection tools is the classification of norovirus as a category B bioterrorism agent by the Centers for Disease Control and Prevention. Among the various potential environmental monitoring matrixes of interest, stool likely represents the most important and arguably the most challenging matrix, due to the abundance and diversity of microorganisms, cell debris, proteins, and lipids contained therein (15). The complexity of stool suggests that one or more purification steps likely are required to extract and enrich the viral fraction prior to analysis, regardless of the type of assay chosen.
Methods for accurate measurement of enteric viruses in environmental matrixes remain elusive, aside from those developed for shellfish and large volumes of water (25, 27). One important challenge to the detection of infectious human noroviruses is the limited culturability of this pathogen, which cannot be grown in tissue cultures or hosts other than humans. Detection has traditionally utilized electron microscopy (11). More recent methods include antigen-directed enzyme-linked immunosorbent assays (20) or reverse transcription PCR (RT-PCR) (26, 27). Each of these methods has its own unique challenges, such as target inhibition (PCR), cross-reactivity (enzyme-linked immunosorbent assays), and lengthy sample preparation procedures (electron microscopy), which hinder its effectiveness. As a result, different methods often produce conflicting results (20). The development of a robust, and at the same time rapid, method for virus detection is desirable, because it potentially may allow for a faster and more informed public health response, particularly during outbreak situations.
Mass spectrometry (MS) has been proposed as a method for the detection of viruses in a number of settings (6, 28, 35). With recent advances in MS techniques, the ability to detect specific markers in complex samples has been achieved (7). MS typically targets a highly abundant protein in microorganisms of interest (reviewed in reference 1). For viruses, this target is typically a capsid protein (28, 35). Ionization of the selected target proteins frequently is achieved using either matrix-assisted laser desorption ionization (MALDI) or electrospray ionization techniques. In MALDI, the sample is cocrystallized with an energy-absorbing matrix, such as 2,5-dihydroxybenzoic acid, and immobilized on a stainless-steel target plate (1). A laser is then fired upon the crystals, which causes ionization of proteins and peptides. Due to the physics of the interaction, predominantly singly charged ions are produced and ejected (9). MALDI is usually coupled with a time-of-flight (TOF) detector, which aptly measures the time taken for a charged molecule (ion) to travel down a flight tube in a vacuum toward an opposing charge, the travel time being a function of the mass-to-charge ratio (m/z) of the analyte (8). Alternatively, in electrospray ionization MS, the sample is presented in a liquid solvent which is sprayed through a fine-capillary tube that is impinged upon by a powerful high-voltage (≥2-kV) electric field (29). The solvent contained in the resultant charged droplets can be blown off, leaving behind ions which are then electromagnetically focused and detected by MS. In contrast to MALDI, electrospray ionization produces predominantly multiply charged ions (34). The sensitivity of this assay can be improved by using nanospray sample introduction techniques. Both MALDI and electrospray ionization are effective tools for the ionization of peptides for mass spectrometric analysis. Comparative studies have demonstrated the pros and cons of both methods (22).
The present study employed MALDI-TOF and electrospray ionization MS (i) to identify biomarkers for norovirus, (ii) to demonstrate the possibility of norovirus detection using three different proteomic MS approaches, (iii) to determine the limit of detection of MALDI-TOF MS using virus-like particles (VLPs) of defined purity and quantity as a surrogate for the actual infective target, (iv) to evaluate a rapid protocol for the efficient purification of virus particles from human stool, and (v) to explore the potential of MS for accurate detection of biomarkers for norovirus in a complex sample matrix, i.e., in fortified extracts of clinical stool samples.
MATERIALS AND METHODS
Experimental design.
Three different analytical approaches were tested to evaluate the possibility of norovirus detection by mass spectrometry. First, authentic standards of intact capsid protein of recombinant norovirus VLPs were scanned in the high-mass range (m/z 25,000 to 200,000; linear mode). Second, the applicability of peptide mass fingerprinting was determined by scanning for characteristic peptides in the low-mass range (m/z 700 to 5,000 in reflectron mode) following tryptic digestion of VLPs contained in authentic standards and in spiked performance samples (i.e., the operationally defined “stool extract”). Finally, peptide sequencing by tandem mass spectrometry was evaluated as a tool for improving target identification in complex samples. In addition, a procedure for virus extraction and concentration was selected and its efficacy determined using bacteriophage MS2 as a culturable surrogate for norovirus. Detection limits for virus protein and particles were determined by spiking known quantities of these targets into both clean and complex matrixes prior to analysis. The latter process ensured that the true amount of protein in the sample was known regardless of the amount of interferences contained therein.
Virus-like particles and microorganisms.
Samples of recombinant norovirus virus-like particles, kindly provided by Mary Estes, were stored in phosphate-buffered saline at 4°C. The VLP stock contained approximately 3.5 mg/ml of protein, which corresponds to 2 × 1011 VLPs (635 picomoles of VLP capsid protein) per microliter, as measured by the bicinchoninic acid method following the manufacturer's instructions (Pierce, Rockford, IL). Stock purity was ascertained using gel electrophoresis followed by in-gel digestion and protein identification using MS (data not shown). Authentic standards of VLPs prepared for mass spectral analysis consisted of diluted VLP stock. MS2 bacteriophage (ATCC 15597B1) and Escherichia coli C3000 (ATCC 15597) were obtained from the American Type Culture Collection, Manassas, VA.
RT-PCR assay.
Stool samples were assayed with molecular diagnostic techniques for the presence of norovirus using heat release of RNA from virus particles followed by RT-PCR as described previously (4).
Bacteriophage cultivation and enumeration.
Stocks of MS2 bacteriophage (ATCC 15597B1) were generated by inoculating E. coli C3000 (ATCC 15597) grown to log phase in 3% tryptic soy broth with MS2 bacteriophage at a 0.1 multiplicity of infection (31). Following overnight incubation at 37°C with shaking at 250 rpm, viral progeny were subsequently purified by extraction with an equal volume of Vertrel XF (DuPont, Wilmington, DE) and stored at −80°C. Phages were enumerated by the soft-agar overlay method (31). Briefly, the virus sample and bacterial host were added to a tube containing 3 ml of soft agar and mixed, and the suspension was poured onto bottom-agar plates. Following solidification, the plates were inverted and incubated at 37°C overnight, and resulting plaques were counted.
In silico digestions and target identification.
The target protein selected for analysis was a synthetic construct (NCBI accession number gi|34223984) of the Norwalk virus capsid protein. The protein has a mass of 56,196 Da (527 amino acids) and a pI value of 5.6. In silico digestions were conducted with Protein Prospector (http://prospector.ucsf.edu) using the following selection of parameters: trypsin; mass range of 700 to 5,000 Da; zero missed cleavages; and unmodified cysteine residues. A variable modification of methionine oxidation was also entered, as this is a common occurrence during sample preparation. The remaining variables were left at their default values.
Sample preparation.
Virus-free stool (1 g) from clinical control samples was suspended in phosphate-buffered saline (Invitrogen, Carlsbad, CA), and in some instances bacteriophage MS2 was added for the determination of virus recovery rates. These diluted stool samples were extracted using established protocols (14, 18, 19) with slight modifications to obtain a sample fraction enriched in virus particles and depleted in nontarget proteins and cell debris. Briefly, an equal volume of Vertrel XF was added to remove lipids and other interferences. The mixture was vortexed for 3 min and centrifuged (3,000 × g; 10 min). Subsequently, the aqueous phase was removed and passed through a 0.22-μm syringe filter (Millipore, Bedford, MA). The filtrate was retained and passed through a 100-kDa-molecular-mass-cutoff, low-protein-binding spin filter (Millipore, Bedford, MA). The retentate (filter cake) was washed with 40 ml of 50 mM ammonium bicarbonate buffer, and the purified sample constituents were suspended in 100 μl of 50 mM NH4HCO3. The obtained operationally defined “stool extract” was either analyzed for bacteriophage presence using the techniques described above or fortified with recombinant VLPs and subjected to mass spectrometric analysis by MALDI-TOF MS and nanospray tandem mass spectrometry. VLPs were added to the “stool extract” rather than to the stool to ensure the presence of the target protein in the sample at a known concentration.
Analysis of intact virus capsid protein by MALDI-TOF MS.
Capsid protein (∼60 picomoles) was dissolved in acetonitrile (50%) containing 10 mg/ml sinapinic acid and 0.1% trifluoroacetic acid and deposited onto a 100-well stainless-steel target plate (Applied Biosystems, Foster City, CA). Samples were analyzed using a Voyager DE-STR MALDI TOF MS with a laser power of 2,700 arbitrary units and an accelerating voltage of 25 kV. External calibration was conducted using a standard protein mixture.
Analysis of digested protein by peptide mass fingerprinting using MALDI-TOF MS.
Standards and stool extracts were vacuum dried, mixed with 200 ng trypsin in 50 mM NH4HCO3, and incubated at 37°C. Trypsin activity was halted via the addition of 10 to 20 μl of 0.1% trifluoroacetic acid. Samples were cleaned and desalted using C18 Omix microextraction columns (Varian, Palo Alto, CA), following the manufacturer's instructions. After a rinse, the retained peptides were suspended in a matrix solution (10 mg/ml of 2,5-dihydroxybenzoic acid or saturated α-cyano-4-hydroxycinnamic acid in an 80:20 mix of acetonitrile and 0.1% trifluoroacetic acid) and deposited on the target plate. Samples were analyzed on the Voyager MS in reflector mode using bradykinin 1-7, ACTH clip 18-39, and oxidized insulin B-chain (Sigma, St. Louis, MO) for external calibration or trypsin autolysis fragments for internal calibration.
Peptide sequencing by tandem mass spectrometry.
Peptide sequencing was performed using a QSTAR Pulsar quadrupole orthogonal TOF tandem mass spectrometer (Applied Biosystems/MDX Sciex; Foster City, CA). Peptides were nanosprayed through a 1-μm tip (Proxeon Bioscience, Denmark) into the QSTAR and fragmented using adjusting collision energies for maximum fragmentation. Fragmentation spectra were accumulated in the multichannel acquisition mode with 3-s durations. Errors on fragmentation ions were less than 0.02 Da. Unspiked stool extract served as a negative control.
Mass spectral data reduction and interpretation.
Ion lists of the most intense peaks (25 to 300 peaks having an intensity of >5% of the most prominent ion) were generated from monoisotopic mass spectra and searched against the entire NCBI nonredundant database (NCBInr; http://www.ncbi.nlm.nih.gov) using Mascot (http://www.matrixscience.com) peptide mass fingerprinting and peptide sequencing tools (16). The searches specified trypsin as the cleavage enzyme. Missed cleavages were never allowed, because this practice did not improve the overall performance of the method. Oxidation of methionine was selected as a variable modification. The mass error was 100 ppm for peptide mass fingerprinting searches and 0.6 Da for tandem MS queries.
RESULTS
In silico analyses.
Theoretical digestion of the norovirus capsid protein with porcine trypsin (EC 3.4.21.4) generated 16 distinct peptides in the analysis range (m/z 700 to 5,000) and 7 potential sites of methionine oxidation (Table 1). The maximum theoretical protein coverage was calculated to equal 57%, assuming no missed cleavages in the trypsin digestion and successful detection by MALDI-TOF MS of all anticipated target peptides in the monitoring range.
TABLE 1.
Protein coverage, amino acid sequences, and corresponding theoretical masses of peptides of the Norovirus virus-like particle capsid protein (accession no. gi|34223984)a,d
| Theoretical massb (M+H) | No. of amino acids | % Coverage of total protein | Amino acid sequence |
|---|---|---|---|
| 707.4 | 6 | 1.1 | NLGEFK |
| 777.4 | 7 | 1.3 | NQQTMRc |
| 1,128.6 | 9 | 1.7 | NVLFHNNDR |
| 1,193.6 | 12 | 2.3 | IMLAGNAFTAGK |
| 1,208.7 | 10 | 1.9 | LVCMLYTPLR |
| 1,280.6 | 14 | 2.7 | TGGGTGDSFVVAGR |
| 1,408.8 | 14 | 2.7 | LVGTTPVSLSHVAK |
| 1,495.8 | 13 | 2.5 | TLDPIEVPLEDVR |
| 1,524.8 | 14 | 2.7 | FYQLKPVGTASSAR |
| 2,000.1 | 18 | 3.4 | TRPFTLPNLPLSSLSNSR |
| 2,509.3 | 22 | 4.2 | VMTCPSPDFNFLFLVPPTVEQK |
| 2,542.3 | 24 | 4.6 | APLPISSMGISPDNVQSVQFQNGR |
| 3,286.8 | 31 | 5.9 | IIVSCIPPGFGSHNLTIAQATLFPHVIADVR |
| 3,555.8 | 33 | 6.3 | IPNYGSSITEATHLAPSVYPPGFGEVLVFFMSK |
| 3,709.8 | 35 | 6.6 | AYPDGFLTCVPNGASSGPQQLPINGVFVFVSWVSR |
| 4,691.3 | 44 | 8.3 | MPGPGAYNLPCLLPQEYISHLASEQAPTVGEAALLHYVDPDTGR |
| 33,021 (of 56,196 total) | 527 | 57a (19) |
Obtained by in silico tryptic digestion; peptide masses outside of the monitoring range (m/z 700 to 5,000) were omitted.
All (M+H) values represent monoisotopic masses.
Potential sites of methionine oxidation (m/z +16) are identified by underscoring.
Peptide masses detected experimentally during analysis of 100 femtomoles of capsid protein by MALDI-TOF MS are shown in bold, along with empirical protein coverage.
Detection limits in clean matrix.
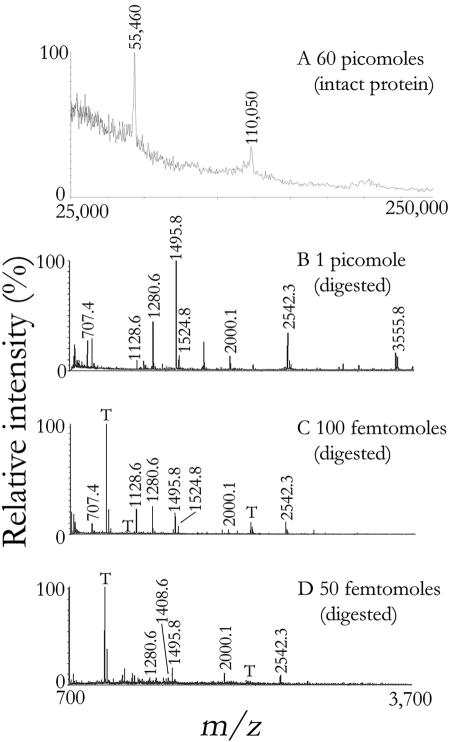
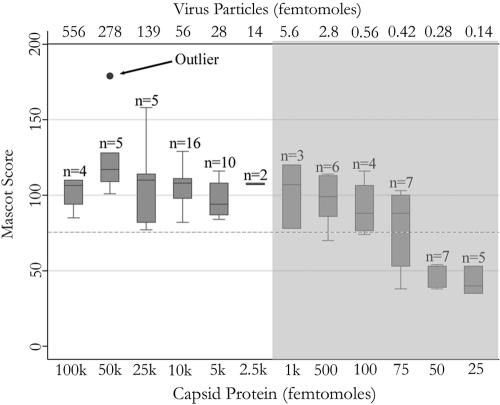
Using the analysis strategy shown in Fig. 1, monomers and dimers of the 56-kDa norovirus capsid protein were detectable by scanning in the high-mass range when present in picomole quantities (1 pmol equals 10−12 moles); observed discrepancies between theoretical (m/z 56,196) and observed (m/z 55,460) masses likely were due to unidentified protein modifications (Fig. 2A). By comparison, peptide mass fingerprinting yielded better sensitivity and better selectivity, thereby allowing the detection of target peptides from as little as 50 femtomoles of protein (1 fmol equals 10−15 moles). Representative mass spectra obtained for three different protein amounts were dominated by high-intensity ions at m/z 1,495.8 and 2,542.3 (Fig. 2B to D). Using the entire NCBInr database (all taxa), significant scores of >77 were consistently obtained (P = 0.05) (Fig. 3). An amount of 50 pmol of capsid protein yielded the highest score of 179, corresponding to a total of 15 unmodified and one modified peptide (52% protein coverage). Aside from this outlier, both the score and protein coverage reached a plateau in the low picomolar range, and no benefit was observable upon further increasing the amount of target protein in the samples. The capsid protein was confidently identified down to 100 fmol, in which case seven peptides were found (Table 1). Below 100 fmol, protein identification was not always reproducible (Fig. 3).
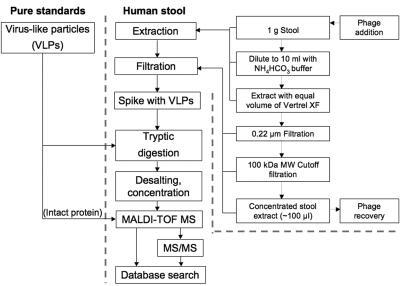
FIG. 1.
Flow chart of the study design and experimental procedures. Pure standards of VLPs were serially diluted to various concentrations, digested with trypsin, and subsequently analyzed by MALDI-TOF MS. The virus extraction and concentration process shown to the right takes 2 to 3 h and reduces an initial quantity of 1 g of stool to a 100-μl concentrate (“stool extract”) enriched in particles having dimensions of intact virus particles (>100 kDa). When MALDI-TOF MS was insufficient to unambiguously identify VLPs, i.e., during analysis of stool extract, confirmatory analysis by nanospray tandem MS (ms/ms) was conducted.
FIG. 2.
Mass spectrometric analyses of authentic standards of VLPs. Undigested VLPs were analyzed by MALDI-TOF MS via scanning of intact proteins. Two peaks corresponding to the monomers (m/z 55,460) and dimers (m/z 110,050) of capsid protein were observed (A). Tryptic digests of various amounts of VLPs were cocrystallized with 2,5-dihydroxybenzoic acid and analyzed by MALDI-TOF MS (B, C, D). Collected spectra were calibrated externally (B) or internally using trypsin autolysis peaks (“T”) where possible (C, D). Target peaks are labeled with their characteristic m/z ratios.
FIG. 3.
Box plot showing the sensitivity of the MALDI-TOF MS analysis. The Mascot score is derived from a probability-based algorithm; the greater the score, the higher the probability that the identification is accurate. The dashed line represents the significance cutoff of P = 0.05 for the entire NCBI nonredundant database searched (20051110 NCBInr; 3,023,944 entries). Data are derived from a total of 74 experiments using either 2,5-dihydroxybenzoic acid or α-cyano-4-hydroxycinnamic acid as the ionization matrix. The shaded area represents published clinical levels of norovirus titers measured per 1 ml of stool using conventional techniques (21).
Recovery of virus from stool samples.
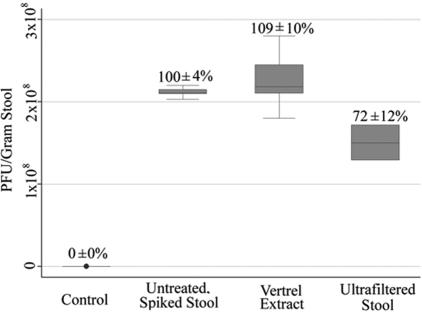
To assess the efficiency of viral recovery and concentration from stool during the various processing steps employed (Fig. 1), the stool sample was seeded with bacteriophage MS2, a culturable virus resembling recombinant Norwalk virus VLPs in size and structure. Aliquots were taken throughout the process to monitor for loss of this VLP surrogate. Recovery rates determined by the double-layer plaque assay (31) demonstrated an overall efficiency of virus particle extraction and concentration of 72% ± 12% (n = 3), with the majority of losses attributable to the filtration step employing a 100-kDa-molecular-mass-cutoff filter (Fig. 4).
FIG. 4.
Assessment of virus recovery during stool sample processing. Bacteriophage MS2, a structurally similar and culturable surrogate for the nonculturable norovirus, was spiked into bacteriophage-negative stool, and aliquots were taken along the sample-processing path (see Fig. 1). Recovery was assessed using the metric of PFU per gram of stool. Error bars represent the 95% confidence interval.
Detection of VLPs in stool matrix.
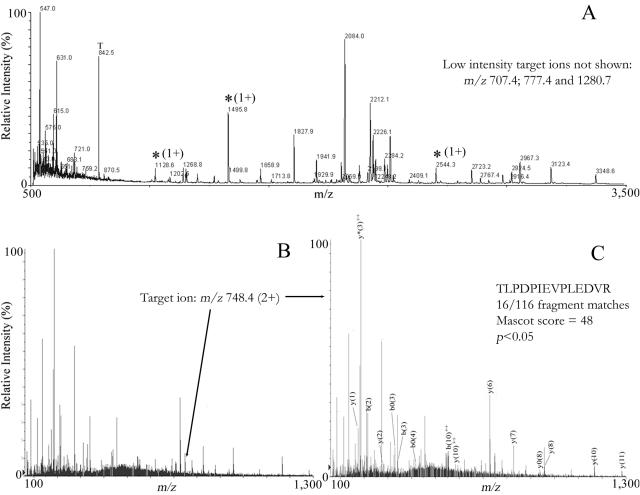
The applicability of mass spectrometry for the detection of virus particles in a complex matrix was assessed using human stool void of both MS2 bacteriophage and norovirus, as demonstrated by the double-layer plaque assay (31) and RT-PCR (4), respectively. Aliquots of stool were processed as shown in Fig. 1. Controls consisting of nonspiked stool extract did not produce any significant protein identifications using peptide mass fingerprinting (spectra not shown). Analysis of stool extract fortified with VLPs to final capsid protein levels of 0.25 to 10 pmol per sample never yielded significant results using peptide mass fingerprinting, although a number of characteristic ions were detected in all spiked samples examined. Even at the lowest level tested (250 fmol of capsid protein), three high-intensity target peaks (m/z 1,128.6, 1,495.8, and 2,544.3) were observed (Fig. 5A). Three other peptides corresponding to the capsid protein were present at lower intensities (m/z 707.4, 777.4, and 1,280.7). The limited number of target peptides and the large number of interferences prevented a successful identification using Mascot peptide mass fingerprinting database searches. Therefore, the high-intensity ion at m/z 1,495.8 was selected for peptide sequencing using collision-induced dissociation tandem mass spectrometry. Nanospray spectra showed the doubly charged ion of the 1,495.8-Da peptide (Fig. 5B; m/z 748.4). Collision-induced dissociation spectra of the mass at m/z 748.4 resulted in the production of 16 fragments that, when queried using the Mascot MS/MS tool, identified the amino acid sequence as TLPDPIEVPLEDVR from norovirus capsid protein (Fig. 5C) (Mascot score of 48; P < 0.05). The predominant ions were from the y series, with 8 out of 12 fragment ions observed. The remaining ions were from the b, b0, y0, b2+, and y2+ series.
FIG. 5.
Representative mass spectra of human stool extract spiked with 250 fmol of capsid protein (∼1.4 fmol of VLPs). MALDI-TOF MS (A) revealed three ions (*) corresponding to target peptides. Based upon relative intensity, the mass at m/z 1,495.8 was selected for peptide sequencing by nanospray tandem MS. This alternative ionization method yielded a doubly charged peptide (M+H2)2+ at m/z 748.4 (B), whose tandem MS spectrum (C) was identified with high confidence (P < 0.05) as TLDPIEVPLEDVR (2% sequence coverage, 16/116 fragments identified) by Mascot database searches of the NCBInr database.
DISCUSSION
The role of mass spectrometry in virus identification.
Mass spectrometry recently has been utilized as a tool for rapid identification of microorganisms in a variety of settings (6, 12, 17), and it is regarded as an important technique for the detection of bioterrorism agents, particularly in air (10, 24). However, use of MS for identification of viruses in complex matrixes is still in its infancy. Virus capsid proteins offer excellent targets for proteomic screening, since a single virus particle often contains multiple copies of characteristic proteins that are readily accessible to proteolytic digestion (28, 35). Proteolysis of target biomarkers yields more information than analysis of intact viral proteins, as demonstrated here by the data shown in Fig. 2. Protein digestion increases the number of characteristic biomarkers and also provides multiple targets for subsequent tandem MS analysis, which can aid in unambiguous identification, particularly in difficult sample matrixes (Fig. 5). The present study shows that MALDI-TOF MS can serve as a rapid and inexpensive screening tool for relatively clean samples, whereas the more elaborate and expensive nanospray analysis may be required for more difficult matrixes and for confirmatory identification.
Selection of viral target and biomarker.
The present investigation concentrated on norovirus detection for several reasons. Noroviruses are important public health threats for which rapid detection techniques are direly needed, in part because these organisms potentially could be used as biological weapons. According to the classification scheme of the U.S. Centers for Disease Control and Prevention, biological agents can be divided into three categories. Category A agents, such as Bacillus anthracis, are those that can be disseminated easily to many people in a given area, with the potential for causing a major public health impact. Category B agents, including Cryptosporidium, noroviruses, and Mycobacterium tuberculosis, are moderately easy to spread and would be expected to result in low mortality rates compared to category A agents. Category C agents include emerging pathogens, such as hantavirus, that potentially could be engineered in the future for mass dissemination because of availability and ease of production (23). Noroviruses also offer some structural features that make them attractive for mass spectrometric analysis. Infective noroviruses and the recombinant, noninfective surrogates used in this study are composed of only a single major structural protein, of which 180 copies join together to form the self-assembled virus capsid. This basic structural proteome of one simplifies mass spectrometric analysis, as it offers a default target of relatively high abundance.
Sensitivity of MALDI-TOF MS.
Use of MALDI-TOF MS was suitable for identifying the target protein confidently and reproducibly at a detection limit of 100 femtomoles. Since each VLP is composed of 180 capsid proteins, the equivalent detection limit for VLPs in authentic standards was 560 attomoles (1 attomole equals 10−18 moles), corresponding to a titer of approximately 3 × 108 viruses per sample. In the sensitivity analysis, the Mascot score was demonstrated to be independent of protein mass at levels of ≥500 fmol of capsid protein. Below 500 fmol, a linear trend was observed relating the score to virus abundance, with a coefficient of determination (r2 value) of approximately 0.7 (data not shown). However, the methods evaluated here were considered to be primarily of a qualitative and not a quantitative nature.
Sample processing and detection of VLPs in stool.
When dealing with a stool sample of an infected individual, the target protein of the norovirus, or any other virus for that matter, will be present in a mixture composed of a large number of proteins originating from the human host, commensal bacteria, and food (15). This obviously complicates the analysis, as a given viral target must be concentrated in an interference-depleted fraction of the processed stool. Typical virus isolation protocols utilize dialysis and sucrose gradient ultracentrifugation, which can take several days to complete (30). The here-examined alternative method for virus extraction and purification, which is based on published techniques (14, 18, 19), was found to be fast (2 to 3 h from start to finish), utilized only inexpensive physical fractionation steps, and potentially can be fully automated. Although this protocol avoids ultracentrifugation, dialysis, gel electrophoresis, and other time-consuming cleanup steps, it removed interferences sufficiently to allow for mass spectrometric detection of virus particles at levels approaching clinically relevant concentrations (Fig. 5).
Tandem mass spectrometric analysis facilitated successful detection of 250 fmol of capsid protein contained in VLPs spiked into the stool extract. Confident identification was achieved using a single target peptide (P < 0.05); attempts to sequence other target peptides were hindered by poor ionization. Use of a sequencing MALDI mass spectrometer likely would have been beneficial in increasing the protein coverage by peptide sequencing. The detection level (250 fmol) corresponds to approximately 8.5 × 108 viruses per ml of stool. An individual infected with norovirus typically sheds 0.1 × 107 to 5 × 107 viruses per ml (0.3 to 15 fmol) during the acute phase, although there have been reports of up to 1010 viruses per ml stool (21). The virus recovery rate of the sample preparation procedure presented here was approximately 70%. Therefore, successful identification of the virus in clinical samples with the method presented here is expected to require processing of a minimum of 125 μl of stool containing a high titer of viruses (i.e., 1010 per ml). Successful pathogen detection in samples containing fewer viruses will require any one or a combination of the following modifications to the presented protocol: (i) processing of larger volumes of specimens, (ii) enhanced virus purification/concentration procedures, or (iii) mass spectrometers featuring greater sensitivity, selectivity, and mass resolution.
Toward MS-based identification of pathogens.
Identification of the causative agent in an outbreak situation is important in order to best direct public health responses and to identify at-risk individuals. With respect to the identification of a disease-causing agent, norovirus presents an exceptional challenge due to the lack of in vitro methods for culturing the pathogen. Methods using RT-PCR may result in false-negative identifications due to PCR inhibitors in the sample (2). Electron microscopy and visual confirmation of virus particles in stool may yield detection limits superior to those reported here. However, electron microscopy does not provide information on certain structural details, such as virus-specific amino acid sequences; in addition, successful detection is reliant on the expertise and experience of the technician or clinician performing the assay (11, 20). Mass spectrometry, in contrast, can be automated, is operator independent (7), and also may yield important information on the type of virus detected, i.e., virus-specific peptide sequences. Although the sensitivity of the MS-based assay is much more limited than that of RT-PCR, the procedure operates just within the clinical range, as demonstrated here (100 to 1,000 fmol) (Fig. 3).
This is the first report of successful identification of norovirus proteins by mass spectrometry. By exploring three different MS-based detection strategies, the study served to highlight the potential and limitations of intact protein analysis, peptide mass fingerprinting, and peptide sequencing for the detection of viruses in clean and more-complex sample matrixes. Based on the results presented here, detection of viral pathogens extracted from human stool and similar environmental matrixes, such as waste from concentrated animal feeding operations, appears promising but will require significant additional research. Such future efforts are warranted, because MS-based techniques potentially could aid in disease diagnosis, outbreak control, epidemiology, and counterterrorism.
Acknowledgments
We thank Mary Estes for providing the recombinant Norwalk virus virus-like particles. We appreciate Kristen Gibson's assistance with virus extraction from clinical samples, and we thank Dawn Zhaohui Chen for her assistance with tandem mass spectrometric analyses. We thank Chadwick Bradford for helping with the MALDI analysis.
David Colquhoun was supported in part by a predoctoral fellowship from the Johns Hopkins Center for a Livable Future. This research was made possible in part by the National Institute of Environmental Health Sciences, through the Johns Hopkins University Center in Urban Environmental Health (P30ES03819), and by a pilot project grant from the Johns Hopkins Center for a Livable Future.
REFERENCES
- 1.Bakhtiar, R., and F. L. Tse. 2000. Biological mass spectrometry: a primer. Mutagenesis 15:415-430. [DOI] [PubMed] [Google Scholar]
- 2.Bon, F., H. Giraudon, C. Sancey, C. Barranger, M. Joannes, P. Pothier, and E. Kohli. 2004. Development and evaluation of a new commercial test allowing the simultaneous detection of noroviruses and sapoviruses by reverse transcription-PCR and microplate hybridization. J. Clin. Microbiol. 42:2218-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2003. Norovirus: technical fact sheet. [Online.] Centers for Disease Control and Prevention, Atlanta, Ga. http://www.cdc.gov/ncidod/dvrd/revb/gastro/noro-factsheet.pdf.
- 4.Chapin, A. R., C. M. Carpenter, W. C. Dudley, L. C. Gibson, R. Pratdesaba, O. Torres, D. Sanchez, J. Belkind-Gerson, I. Nyquist, A. Karnell, B. Gustafsson, J. L. Halpern, A. L. Bourgeois, and K. J. Schwab. 2005. Prevalence of norovirus among visitors from the United States to Mexico and Guatemala who experience traveler's diarrhea. J. Clin. Microbiol. 43:1112-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 6.Fenselau, C., and P. A. Demirev. 2001. Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrom. Rev. 20:157-171. [DOI] [PubMed] [Google Scholar]
- 7.Halden, R. U., D. R. Colquhoun, and E. S. Wisniewski. 2005. Identification and phenotypic characterization of Sphingomonas wittichii strain RW1 by peptide mass fingerprinting using matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 71:2442-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillenkamp, F., and M. Karas. 1990. Mass spectrometry of peptides and proteins by matrix-assisted ultraviolet laser desorption/ionization. Methods Enzymol. 193:280-295. [DOI] [PubMed] [Google Scholar]
- 9.Karas, M., M. Gluckmann, and J. Schafer. 2000. Ionization in matrix-assisted laser desorption/ionization: singly charged molecular ions are the lucky survivors. J. Mass Spectrom. 35:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Kim, J. K., S. N. Jackson, and K. K. Murray. 2005. Matrix-assisted laser desorption/ionization mass spectrometry of collected bioaerosol particles. Rapid Commun. Mass Spectrom. 19:1725-1729. [DOI] [PubMed] [Google Scholar]
- 11.Lin, Y. P., K. Nicholas, F. R. Ball, B. McLaughlin, and F. R. Bishai. 1991. Detection of Norwalk-like virus and specific antibody by immune-electron microscopy with colloidal gold immune complexes. J. Virol. Methods 35:237-253. [DOI] [PubMed] [Google Scholar]
- 12.Mandrell, R. E., L. A. Harden, A. Bates, W. G. Miller, W. F. Haddon, and C. K. Fagerquist. 2005. Speciation of Campylobacter coli, C. jejuni, C. helveticus, C. lari, C. sputorum, and C. upsaliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 71:6292-6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maunula, L., S. Kalso, C. H. Von Bonsdorff, and A. Ponka. 2004. Wading pool water contaminated with both noroviruses and astroviruses as the source of a gastroenteritis outbreak. Epidemiol. Infect. 132:737-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendez, I. I., L. L. Hermann, P. R. Hazelton, and K. M. Coombs. 2000. A comparative analysis of Freon substitutes in the purification of reovirus and calicivirus. J. Virol. Methods 90:59-67. [DOI] [PubMed] [Google Scholar]
- 15.Oleksiewicz, M. B., H. O. Kjeldal, and T. G. Kleno. 2005. Identification of stool proteins in C57BL/6J mice by two-dimensional gel electrophoresis and MALDI-TOF mass spectrometry. Biomarkers 10:29-40. [DOI] [PubMed] [Google Scholar]
- 16.Pappin, D. J. C., P. Hojrup, and A. J. Bleasby. 1993. Rapid identification of proteins by peptide-mass fingerprinting. Curr. Biol. 3:327-332. [DOI] [PubMed] [Google Scholar]
- 17.Pribil, P. A., E. Patton, G. Black, V. Doroshenko, and C. Fenselau. 2005. Rapid characterization of Bacillus spores targeting species-unique peptides produced with an atmospheric pressure matrix-assisted laser desorption/ionization source. J. Mass Spectrom. 40:464-474. [DOI] [PubMed] [Google Scholar]
- 18.Queiroz, A. P., F. M. Santos, A. Sassaroli, C. M. Harsi, T. A. Monezi, and D. U. Mehnert. 2001. Electropositive filter membrane as an alternative for the elimination of PCR inhibitors from sewage and water samples. Appl. Environ. Microbiol. 67:4614-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quigley, J. J. 1949. Ultrafiltration and ultracentrifugation studies of Coxsackie virus. Proc. Soc. Exp. Biol. Med. 72:434. [DOI] [PubMed] [Google Scholar]
- 20.Rabenau, H. F., M. Sturmer, S. Buxbaum, A. Walczok, W. Preiser, and H. W. Doerr. 2003. Laboratory diagnosis of norovirus: which method is the best? Intervirology 46:232-238. [DOI] [PubMed] [Google Scholar]
- 21.Richards, G. P., M. A. Watson, and D. H. Kingsley. 2004. A SYBR green, real-time RT-PCR method to detect and quantitate Norwalk virus in stools. J. Virol. Methods 116:63-70. [DOI] [PubMed] [Google Scholar]
- 22.Roda, A., A. M. Gioacchini, R. Seraglia, M. Montagnani, M. Baraldini, S. Pedrazzini, M. Puricelli, and P. Traldi. 1997. A comparison of the analytical performance of sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrospray and matrix-assisted laser desorption ionization mass spectrometry in the study of the protein extract from Bothrops jararaca snake venom. Rapid Commun. Mass Spectrom. 11:1297-1302. [Google Scholar]
- 23.Rotz, L. D., A. S. Khan, S. R. Lillibridge, S. M. Ostroff, and J. M. Hughes. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell, S. C., G. Czerwieniec, C. Lebrilla, P. Steele, V. Riot, K. Coffee, M. Frank, and E. E. Gard. 2005. Achieving high detection sensitivity (14 zmol) of biomolecular ions in bioaerosol mass spectrometry. Anal. Chem. 77:4734-4741. [DOI] [PubMed] [Google Scholar]
- 25.Schwab, K. J., F. H. Neill, M. K. Estes, T. G. Metcalf, and R. L. Atmar. 1998. Distribution of Norwalk virus within shellfish following bioaccumulation and subsequent depuration by detection using RT-PCR. J. Food Prot. 61:1674-1680. [DOI] [PubMed] [Google Scholar]
- 26.Schwab, K. J., F. H. Neill, R. L. Fankhauser, N. A. Daniels, S. S. Monroe, D. A. Bergmire-Sweat, M. K. Estes, and R. L. Atmar. 2000. Development of methods to detect “Norwalk-like viruses” (NLVs) and hepatitis A virus in delicatessen foods: application to a food-borne NLV outbreak. Appl. Environ. Microbiol. 66:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwab, K. J., F. H. Neill, F. Le Guyader, M. K. Estes, and R. L. Atmar. 2001. Development of a reverse transcription-PCR-DNA enzyme immunoassay for detection of “Norwalk-like” viruses and hepatitis A virus in stool and shellfish. Appl. Environ. Microbiol. 67:742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siuzdak, G. 1998. Probing viruses with mass spectrometry. J. Mass Spectrom. 33:203-211. [DOI] [PubMed] [Google Scholar]
- 29.Smith, R. D., J. A. Loo, C. G. Edmonds, C. J. Barinaga, and H. R. Udseth. 1990. New developments in biochemical mass spectrometry: electrospray ionization. Anal. Chem. 62:882-899. [DOI] [PubMed] [Google Scholar]
- 30.Tan, Y. P., T. C. Ling, K. Yusoff, W. S. Tan, and B. T. Tey. 2005. Comparative evaluation of three purification methods for the nucleocapsid protein of Newcastle disease virus from Escherichia coli homogenates. J. Microbiol. 43:295-300. [PubMed] [Google Scholar]
- 31.U.S. Environmental Protection Agency. 2001. Method 1602: male-specific (F+) and somatic coliphage in water by single agar layer (SAL) procedure. EPA 821-R-01-029. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
- 32.U.S. Food and Drug Administration. 2 December 2005, revision date. The Norwalk virus family. [Online.] U.S. Food and Drug Administration. http://vm.cfsan.fda.gov/∼mow/chap34.html.
- 33.Widdowson, M. A., A. Sulka, S. N. Bulens, R. S. Beard, S. S. Chaves, R. Hammond, E. D. Salehi, E. Swanson, J. Totaro, R. Woron, P. S. Mead, J. S. Bresee, S. S. Monroe, and R. I. Glass. 2005. Norovirus and foodborne disease, United States, 1991-2000. Emerg. Infect. Dis. 11:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita, M., and J. B. Fenn. 1984. Electrospray ion-source—another variation on the free-jet theme. J. Phys. Chem. 88:4451-4459. [Google Scholar]
- 35.Yao, Z. P., P. A. Demirev, and C. Fenselau. 2002. Mass spectrometry-based proteolytic mapping for rapid virus identification. Anal. Chem. 74:2529-2534. [DOI] [PubMed] [Google Scholar]