Gut flora influences the hypothalamic-gonadal axis to regulate the pathogenesis of obesity-associated precocious puberty
- PMID: 39572735
- PMCID: PMC11582813
- DOI: 10.1038/s41598-024-80140-8
Gut flora influences the hypothalamic-gonadal axis to regulate the pathogenesis of obesity-associated precocious puberty
Abstract
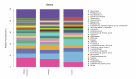
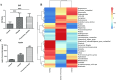
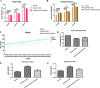
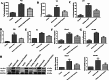
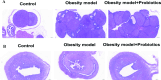
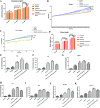
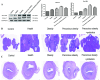
The prevalence of obesity-associated precocious puberty is gradually increasing, but the relationship between gut flora and obesity-associated precocious puberty remains unclear.We analysed the gut flora characteristics of a clinical sample of 30 girls aged 5-8 years using 16s rRNA sequencing. An obesity rat model and a rat model of gut flora transplantation were also constructed. Body weight, body length, abdominal girth, food intake, vulva opening time, and gonadal index were monitored. The secretion levels of estradiol (E2), total cholesterol (TC), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and thyroglobulin (Tg) were analyzed by ELISA. In addition, ovarian and uterine development was observed by HE staining. The mRNA and protein levels of kisspeptin-1 (Kiss-1) and gonadotropin-releasing.We found that the relative abundance of Dialister, Bacteroides, Bifidobacterium, Collinsella, and Romboutsia may be associated with obesity-associated precocious puberty. Obesity promotes gonadal development, and the gut flora of patients with obesity and obesity-associated precocious puberty regulated the gene and protein expression of Kiss-1 and GnRH, promoting precocious puberty and hypothalamic-gonadal axis hormone secretion in rats. In contrast, probiotic intervention slowed gonadal development, reduced hormone secretion, and attenuated hypothalamic-gonadal axis activity. Gut flora promoted obesity-associated precocious puberty by influencing the hypothalamic-gonadal axis, and probiotics have a therapeutic and preventive role in obesity-associated precocious puberty, which may be associated with the Kiss-1/GnRH pathway. These findings may provide some new strategies for clinical treatment and prevention of obesity-associated precocious puberty in girls.
Keywords: Children; Gut flora; Obesity; Precocious puberty.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Study approval statement: This study protocol was reviewed and approved by the Ethics Committee of Jinhua People’s Hospital (No. IBR-20220009-R). Human or animal rights: All participants were informed about the study and signed an informed consent form, and the study was approved by the Ethics Committee of Jinhua People’s Hospital (No. IBR-20220009-R). Consent for publication: The authors declared that all participants consented to the publication of this study.
Figures
References
-
- Gu, Q., Du, Q., Xia, L., et al. Mechanistic insights into EGCG’s preventive effects on obesity-induced precocious puberty through multi-omics analyses (Food Funct, 2024). - PubMed
-
- Krishna K.B. & Silverman, L.A. Diagnosis of central precocious puberty. Endocrinol. Metab. Clin. North. Am.53(2), 217–227 (2024). - PubMed
-
- Huttunen, H. et al. Central precocious puberty in boys: Secular trend and clinical features. Eur. J. Endocrinol.190(3), 211–219 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


