Altitude shapes gut microbiome composition accounting for diet, thyroid hormone levels, and host genetics in a subterranean blind mole rat
- PMID: 39552645
- PMCID: PMC11565052
- DOI: 10.3389/fmicb.2024.1476845
Altitude shapes gut microbiome composition accounting for diet, thyroid hormone levels, and host genetics in a subterranean blind mole rat
Abstract
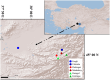
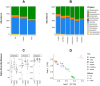
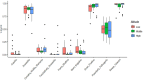
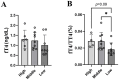
The animal gut microbiome acts as a crucial link between the host and its environment, playing a vital role in digestion, metabolism, physiology, and fitness. Using 16S rRNA metabarcoding, we investigated the effect of altitude on the microbiome composition of Anatolian Blind Mole Rats (Nannospalax xanthodon) across six locations and three altitudinal groups. We also factored in the host diet, as well as host microsatellite genotypes and thyroid hormone levels. The altitude had a major effect on microbiome composition, with notable differences in the relative abundance of several bacterial taxa across elevations. Contrary to prior research, we found no significant difference in strictly anaerobic bacteria abundance among altitudinal groups, though facultatively anaerobic bacteria were more prevalent at higher altitudes. Microbiome alpha diversity peaked at mid-altitude, comprising elements from both low and high elevations. The beta diversity showed significant association with the altitude. Altitude had a significant effect on the diet composition but not on its alpha diversity. No distinct altitude-related genetic structure was evident among the host populations, and no correlation was revealed between the host genetic relatedness and microbiome composition nor between the host microbiome and the diet. Free thyroxine (FT4) levels increased almost linearly with the altitude but none of the bacterial ASVs were found to be specifically associated with hormone levels. Total thyroxine (TT4) levels correlated positively with microbiome diversity. Although we detected correlation between certain components of the thyroid hormone levels and the microbiome beta diversity, the pattern of their relationship remains inconclusive.
Keywords: 16S; 18S; altitude adaptation; blind mole rats; diet; gut microbiome; high altitude; thyroid.
Copyright © 2024 Solak, Kreisinger, Čížková, Sezgin, Schmiedová, Murtskhvaladze, Henning, Çolak, Matur and Yanchukov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources


