New insights for the development of efficient DNA vaccines
- PMID: 39545748
- PMCID: PMC11565620
- DOI: 10.1111/1751-7915.70053
New insights for the development of efficient DNA vaccines
Abstract
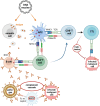
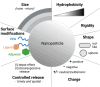
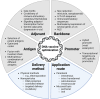
Despite the great potential of DNA vaccines for a broad range of applications, ranging from prevention of infections, over treatment of autoimmune and allergic diseases to cancer immunotherapies, the implementation of such therapies for clinical treatment is far behind the expectations up to now. The main reason is the poor immunogenicity of DNA vaccines in humans. Consequently, the improvement of the performance of DNA vaccines in vivo is required. This mini-review provides an overview of the current state of DNA vaccines and the various strategies to enhance the immunogenic potential of DNA vaccines, including (i) the optimization of the DNA construct itself regarding size, nuclear transfer and transcriptional regulation; (ii) the use of appropriate adjuvants; and (iii) improved delivery, for example, by careful choice of the administration route, physical methods such as electroporation and nanomaterials that may allow cell type-specific targeting. Moreover, combining nanoformulated DNA vaccines with other immunotherapies and prime-boost strategies may help to enhance success of treatment.
© 2024 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest and no competing financial interest.
Figures
References
-
- Afridi, S. , Hoessli, D.C. & Hameed, M.W. (2016) Mechanistic understanding and significance of small peptides interaction with MHC class II molecules for therapeutic applications. Immunological Reviews, 272(1), 151–168. - PubMed
-
- Al‐Deen, F.N. , Selomulya, C. , Ma, C. & Coppel, R.L. (2014) Superparamagnetic nanoparticle delivery of DNA vaccine. Methods in Molecular Biology, 1143, 181–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


