Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates
- PMID: 39460266
- PMCID: PMC11511058
- DOI: 10.3390/vaccines12101099
Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates
Abstract
Background: Influenza and SARS-CoV-2 viruses are two highly variable pathogens. We have developed a candidate bivalent live vaccine based on the strain of licensed A/Leningrad/17-based cold-adapted live attenuated influenza vaccine (LAIV) of H3N2 subtype, which expressed SARS-CoV-2 immunogenic T-cell epitopes. A cassette encoding fragments of S and N proteins of SARS-CoV-2 was inserted into the influenza NA gene using the P2A autocleavage site. In this study, we present the results of preclinical evaluation of the developed bivalent vaccine in a non-human primate model.
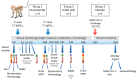
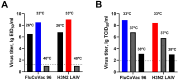
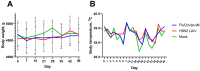
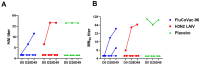
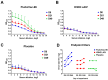
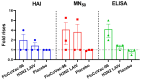
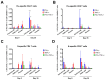
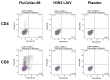
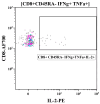
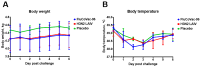
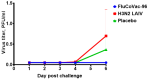
Methods: Rhesus macaques (Macaca mulatta) (n = 3 per group) were immunized intranasally with 7.5 lg EID50 of the LAIV/CoV-2 bivalent vaccine, a control non-modified H3N2 LAIV or a placebo (chorioallantoic fluid) using a sprayer device, twice, with a 28-day interval. The blood samples were collected at days 0, 3, 28 and 35 for hematological and biochemical assessment. Safety was also assessed by monitoring body weight, body temperature and clinical signs of the disease. Immune responses to influenza virus were assessed both by determining serum antibody titers in hemagglutination inhibition assay, microneutralization assay and IgG ELISA. T-cell responses were measured both to influenza and SARS-CoV-2 antigens using ELISPOT and flow cytometry. Three weeks after the second immunization, animals were challenged with 105 PFU of Delta SARS-CoV-2. The body temperature, weight and challenge virus shedding were monitored for 5 days post-challenge. In addition, virus titers in various organs and histopathology were evaluated on day 6 after SARS-CoV-2 infection.
Results: There was no toxic effect of the immunizations on the hematological and coagulation hemostasis of animals. No difference in the dynamics of the average weight and thermometry results were found between the groups of animals. Both LAIV and LAIV/CoV-2 variants poorly replicated in the upper respiratory tract of rhesus macaques. Nevertheless, despite this low level of virus shedding, influenza-specific serum IgG responses were detected in the group of monkeys immunized with the LAIV/CoV-2 bivalent but not in the LAIV group. Furthermore, T-cell responses to both influenza and SARS-CoV-2 viruses were detected in the LAIV/CoV-2 vaccine group only. The animals were generally resistant to SARS-CoV-2 challenge, with minimal virus shedding in the placebo and LAIV groups. Histopathological changes in vaccinated animals were decreased compared to the PBS group, suggesting a protective effect of the chimeric vaccine candidate.
Conclusions: The candidate bivalent vaccine was safe and immunogenic for non-human primates and warrants its further evaluation in clinical trials.
Keywords: SARS-CoV-2; bivalent vaccine; influenza; non-human primates; preclinical study; rhesus monkeys; virus-vectored vaccine.
Conflict of interest statement
Authors I.-I.S., E.S., D.M., V.M. and L.R. have patent #RU 2782531 issued to FSBSI “Institute of experimental medicine”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Development of a T Cell-Based COVID-19 Vaccine Using a Live Attenuated Influenza Vaccine Viral Vector.Vaccines (Basel). 2022 Jul 18;10(7):1142. doi: 10.3390/vaccines10071142. Vaccines (Basel). 2022. PMID: 35891306 Free PMC article.
-
Evaluation of replication, immunogenicity and protective efficacy of a live attenuated cold-adapted pandemic H1N1 influenza virus vaccine in non-human primates.Vaccine. 2012 Aug 17;30(38):5603-10. doi: 10.1016/j.vaccine.2012.06.088. Epub 2012 Jul 10. Vaccine. 2012. PMID: 22789506 Free PMC article.
-
Expression of the SARS-CoV-2 receptor-binding domain by live attenuated influenza vaccine virus as a strategy for designing a bivalent vaccine against COVID-19 and influenza.Virol J. 2024 Apr 9;21(1):82. doi: 10.1186/s12985-024-02350-w. Virol J. 2024. PMID: 38589848 Free PMC article.
-
Safety, Immunogenicity, and Protective Efficacy of a Chimeric A/B Live Attenuated Influenza Vaccine in a Mouse Model.Microorganisms. 2021 Jan 27;9(2):259. doi: 10.3390/microorganisms9020259. Microorganisms. 2021. PMID: 33513862 Free PMC article.
-
Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults.Drugs. 2011 Aug 20;71(12):1591-622. doi: 10.2165/11206860-000000000-00000. Drugs. 2011. PMID: 21861544 Review.
References
-
- Tan A.T., Linster M., Tan C.W., Le Bert N., Chia W.N., Kunasegaran K., Zhuang Y., Tham C.Y.L., Chia A., Smith G.J.D., et al. Early Induction of Functional SARS-CoV-2-Specific T Cells Associates with Rapid Viral Clearance and Mild Disease in COVID-19 Patients. Cell Rep. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. - DOI - PMC - PubMed
-
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. - DOI - PMC - PubMed
-
- Markov N.S., Ren Z., Senkow K.J., Grant R.A., Gao C.A., Malsin E.S., Sichizya L., Kihshen H., Helmin K.A., Jovisic M., et al. Distinctive evolution of alveolar T cell responses is associated with clinical outcomes in unvaccinated patients with SARS-CoV-2 pneumonia. Nat. Immunol. 2024;25:1607–1622. doi: 10.1038/s41590-024-01914-w. - DOI - PMC - PubMed
-
- Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T., Zhu H., Liu J., Xu Y., Xie J., et al. Immunodominant SARS Coronavirus Epitopes in Humans Elicited Both Enhancing and Neutralizing Effects on Infection in Non-Human Primates. ACS Infect. Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


