Continuous Treatment with IncobotulinumtoxinA Despite Presence of BoNT/A Neutralizing Antibodies: Immunological Hypothesis and a Case Report
- PMID: 39453199
- PMCID: PMC11510976
- DOI: 10.3390/toxins16100422
Continuous Treatment with IncobotulinumtoxinA Despite Presence of BoNT/A Neutralizing Antibodies: Immunological Hypothesis and a Case Report
Abstract
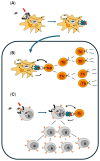
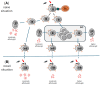
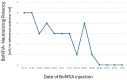
Botulinum Neurotoxin A (BoNT/A) is a bacterial protein that has proven to be a valuable pharmaceutical in therapeutic indications and aesthetic medicine. One major concern is the formation of neutralizing antibodies (nAbs) to the core BoNT/A protein. These can interfere with the therapy, resulting in partial or complete antibody (Ab)-mediated secondary non-response (SNR) or immunoresistance. If titers of nAbs reach a level high enough that all injected BoNT/A molecules are neutralized, immunoresistance occurs. Studies have shown that continuation of treatment of neurology patients who had developed Ab-mediated partial SNR against complexing protein-containing (CPC-) BoNT/A was in some cases successful if patients were switched to complexing protein-free (CPF-) incobotulinumtoxinA (INCO). This seems to contradict the layperson's basic immunological understanding that repeated injection with the same antigen BoNT/A should lead to an increase in antigen-specific antibody titers. As such, we strive to explain how immunological memory works in general, and based on this, we propose a working hypothesis for this paradoxical phenomenon observed in some, but not all, neurology patients with immunoresistance. A critical factor is the presence of potentially immune-stimulatory components in CPC-BoNT/A products that can act as immunologic adjuvants and activate not only naïve, but also memory B lymphocyte responses. Furthermore, we propose that continuous injection of a BoN/TA formulation with low immunogenicity, e.g., INCO, may be a viable option for aesthetic patients with existing nAbs. These concepts are supported by a real-world case example of a patient with immunoresistance whose nAb levels declined with corresponding resumption of clinical response despite regular INCO injections.
Keywords: Botulinum Neurotoxin A; IncobotulinumtoxinA; complexing protein free; immunologic adjuvants; immunological memory; immunoresistance; reactivation; secondary non-response; therapy failure.
Conflict of interest statement
Michael Uwe Martin serves as an ad hoc consultant and speaker for Merz Pharma GmbH & Co., KGaA. Clifton Ming Tay is an employee of Merz Asia Pacific Pte. Ltd. Tuck Wah Siew serves as an ad hoc consultant and speaker for Merz Asia Pacific Pte Ltd.
Figures

Similar articles
-
Complexing Protein-Free Botulinum Neurotoxin A Formulations: Implications of Excipients for Immunogenicity.Toxins (Basel). 2024 Feb 10;16(2):101. doi: 10.3390/toxins16020101. Toxins (Basel). 2024. PMID: 38393178 Free PMC article. Review.
-
IncobotulinumtoxinA: A Highly Purified and Precisely Manufactured Botulinum Neurotoxin Type A.J Drugs Dermatol. 2019 Jan 1;18(1):52-57. J Drugs Dermatol. 2019. PMID: 30681794 Review.
-
Immunogenicity induced by botulinum toxin injections for limb spasticity: A systematic review.Ann Phys Rehabil Med. 2019 Jul;62(4):241-251. doi: 10.1016/j.rehab.2019.03.004. Epub 2019 Apr 11. Ann Phys Rehabil Med. 2019. PMID: 30980953
-
Can clinical and urodynamic parameters predict the occurrence of neutralizing antibodies in therapy failure of intradetrusor onabotulinumtoxin A injections in patients with spinal cord injury?BMC Urol. 2020 Aug 2;20(1):113. doi: 10.1186/s12894-020-00683-6. BMC Urol. 2020. PMID: 32741365 Free PMC article.
-
Immunogenicity Associated with Botulinum Toxin Treatment.Toxins (Basel). 2019 Aug 26;11(9):491. doi: 10.3390/toxins11090491. Toxins (Basel). 2019. PMID: 31454941 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials


