Mitigating Vascular Inflammation by Mimicking AIBP Mechanisms: A New Therapeutic End for Atherosclerotic Cardiovascular Disease
- PMID: 39408645
- PMCID: PMC11477018
- DOI: 10.3390/ijms251910314
Mitigating Vascular Inflammation by Mimicking AIBP Mechanisms: A New Therapeutic End for Atherosclerotic Cardiovascular Disease
Abstract
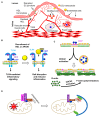
Atherosclerosis, characterized by the accumulation of lipoproteins and lipids within the vascular wall, underlies a heart attack, stroke, and peripheral artery disease. Endothelial inflammation is the primary component driving atherosclerosis, promoting leukocyte adhesion molecule expression (e.g., E-selectin), inducing chemokine secretion, reducing the production of nitric oxide (NO), and enhancing the thrombogenic potential. While current therapies, such as statins, colchicine, anti-IL1β, and sodium-glucose cotransporter 2 (SGLT2) inhibitors, target systemic inflammation, none of them addresses endothelial cell (EC) inflammation, a critical contributor to disease progression. Targeting endothelial inflammation is clinically significant because it can mitigate the root cause of atherosclerosis, potentially preventing disease progression, while reducing the side effects associated with broader anti-inflammatory treatments. Recent studies highlight the potential of the APOA1 binding protein (AIBP) to reduce systemic inflammation in mice. Furthermore, its mechanism of action also guides the design of a potential targeted therapy against a particular inflammatory signaling pathway. This review discusses the unique advantages of repressing vascular inflammation or enhancing vascular quiescence and the associated benefits of reducing thrombosis. This approach offers a promising avenue for more effective and targeted interventions to improve patient outcomes.
Keywords: AIBP; ASCVD; inflamed EC-specific targeting; vascular inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Beta-blockers for Atherosclerosis Prevention: a Missed Opportunity?Curr Atheroscler Rep. 2022 Mar;24(3):161-169. doi: 10.1007/s11883-022-00983-2. Epub 2022 Feb 16. Curr Atheroscler Rep. 2022. PMID: 35174437 Review.
-
AIBP protects against metabolic abnormalities and atherosclerosis.J Lipid Res. 2018 May;59(5):854-863. doi: 10.1194/jlr.M083618. Epub 2018 Mar 20. J Lipid Res. 2018. PMID: 29559522 Free PMC article.
-
Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):790-800. doi: 10.1161/ATVBAHA.113.303116. Epub 2014 Feb 13. Arterioscler Thromb Vasc Biol. 2014. PMID: 24526691
-
Synergistic effects of liposomes encapsulating atorvastatin calcium and curcumin and targeting dysfunctional endothelial cells in reducing atherosclerosis.Int J Nanomedicine. 2019 Jan 15;14:649-665. doi: 10.2147/IJN.S189819. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30697048 Free PMC article.
-
The Role of Sodium Glucose Cotransporter-2 Inhibitors in Atherosclerotic Cardiovascular Disease: A Narrative Review of Potential Mechanisms.Cells. 2021 Oct 9;10(10):2699. doi: 10.3390/cells10102699. Cells. 2021. PMID: 34685677 Free PMC article. Review.
References
-
- Iiyama K., Hajra L., Iiyama M., Li H., DiChiara M., Medoff B.D., Cybulsky M.I. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ. Res. 1999;85:199–207. doi: 10.1161/01.RES.85.2.199. - DOI - PubMed
-
- Zhou Z., Subramanian P., Sevilmis G., Globke B., Soehnlein O., Karshovska E., Megens R., Heyll K., Chun J., Saulnier-Blache J.S., et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011;13:592–600. doi: 10.1016/j.cmet.2011.02.016. - DOI - PubMed
-
- Huang L., Chambliss K.L., Gao X., Yuhanna I.S., Behling-Kelly E., Bergaya S., Ahmed M., Michaely P., Luby-Phelps K., Darehshouri A., et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature. 2019;569:565–569. doi: 10.1038/s41586-019-1140-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


