Integrating amyloid imaging and genetics for early risk stratification of Alzheimer's disease
- PMID: 39285750
- PMCID: PMC11567859
- DOI: 10.1002/alz.14244
Integrating amyloid imaging and genetics for early risk stratification of Alzheimer's disease
Abstract
Introduction: Alzheimer's disease (AD) initiates years prior to symptoms, underscoring the importance of early detection. While amyloid accumulation starts early, individuals with substantial amyloid burden may remain cognitively normal, implying that amyloid alone is not sufficient for early risk assessment.
Methods: Given the genetic susceptibility of AD, a multi-factorial pseudotime approach was proposed to integrate amyloid imaging and genotype data for estimating a risk score. Validation involved association with cognitive decline and survival analysis across risk-stratified groups, focusing on patients with mild cognitive impairment (MCI).
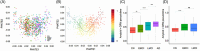
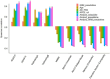
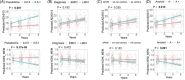
Results: Our risk score outperformed amyloid composite standardized uptake value ratio in correlation with cognitive scores. MCI subjects with lower pseudotime risk score showed substantial delayed onset of AD and slower cognitive decline. Moreover, pseudotime risk score demonstrated strong capability in risk stratification within traditionally defined subgroups such as early MCI, apolipoprotein E (APOE) ε4+ MCI, APOE ε4- MCI, and amyloid+ MCI.
Discussion: Our risk score holds great potential to improve the precision of early risk assessment.
Highlights: Accurate early risk assessment is critical for the success of clinical trials. A new risk score was built from integrating amyloid imaging and genetic data. Our risk score demonstrated improved capability in early risk stratification.
Keywords: Alzheimer's disease; imaging genetics; longitudinal association analysis; pseudotime analysis; survival analysis.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Saykin has received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor) and holds advisory roles with Siemens Medical Solutions USA, Inc., NIH NHLBI, and Eisai. His editorial commitments include serving as editor‐in‐chief for the journal
Figures


Similar articles
-
APOLLOE4 Phase 3 study of oral ALZ-801/valiltramiprosate in APOE ε4/ε4 homozygotes with early Alzheimer's disease: Trial design and baseline characteristics.Alzheimers Dement (N Y). 2024 Aug 13;10(3):e12498. doi: 10.1002/trc2.12498. eCollection 2024 Jul-Sep. Alzheimers Dement (N Y). 2024. PMID: 39144121 Free PMC article.
-
APOE ε4/ε4 homozygotes with early Alzheimer's disease show accelerated hippocampal atrophy and cortical thinning that correlates with cognitive decline.Alzheimers Dement (N Y). 2020 Dec 4;6(1):e12117. doi: 10.1002/trc2.12117. eCollection 2020. Alzheimers Dement (N Y). 2020. PMID: 33304988 Free PMC article.
-
Amyloid-β and APOE genotype predict memory decline in cognitively unimpaired older individuals independently of Alzheimer's disease polygenic risk score.BMC Neurol. 2022 Dec 15;22(1):484. doi: 10.1186/s12883-022-02925-6. BMC Neurol. 2022. PMID: 36522743 Free PMC article.
-
2014 Update of the Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception.Alzheimers Dement. 2015 Jun;11(6):e1-120. doi: 10.1016/j.jalz.2014.11.001. Alzheimers Dement. 2015. PMID: 26073027 Free PMC article. Review.
-
Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative.Alzheimers Dement. 2019 Jan;15(1):106-152. doi: 10.1016/j.jalz.2018.08.005. Epub 2018 Oct 13. Alzheimers Dement. 2019. PMID: 30321505 Review.
References
-
- Gatz M, Pedersen NL, Berg S, et al. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52(2):M117‐M125. - PubMed
-
- Sims R, Hill M, Williams J. The multiplex model of the genetics of Alzheimer's disease. Nat Neurosci. 2020;23(3):311‐322. - PubMed
-
- Organization WH, et al. Risk reduction of cognitive decline and dementia: WHO guidelines 2019. - PubMed
-
- Van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG019771/AG/NIA NIH HHS/United States
- R21AG072101/AG/NIA NIH HHS/United States
- U19AG024904/AG/NIA NIH HHS/United States
- NIH
- U19 AG024904/AG/NIA NIH HHS/United States
- 1942394/National Science Foundation
- R21 AG072101/AG/NIA NIH HHS/United States
- R01AG019771/AG/NIA NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- U01AG072177/AG/NIA NIH HHS/United States
- T32AG071444/AG/NIA NIH HHS/United States
- R01AG068193/AG/NIA NIH HHS/United States
- T32 AG071444/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P30AG072976/AG/NIA NIH HHS/United States
- U01 AG072177/AG/NIA NIH HHS/United States
- U19 AG074879/AG/NIA NIH HHS/United States
- U01AG068057/AG/NIA NIH HHS/United States
- P30AG010133/AG/NIA NIH HHS/United States
- U01 AG068057/AG/NIA NIH HHS/United States
- R01 AG057739/AG/NIA NIH HHS/United States
- R01 AG068193/AG/NIA NIH HHS/United States
- R01AG057739/AG/NIA NIH HHS/United States
- R01 LM013463/LM/NLM NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


