Small LEA proteins mitigate air-water interface damage to fragile cryo-EM samples during plunge freezing
- PMID: 39231985
- PMCID: PMC11375022
- DOI: 10.1038/s41467-024-52091-1
Small LEA proteins mitigate air-water interface damage to fragile cryo-EM samples during plunge freezing
Abstract
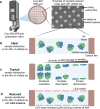
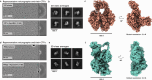
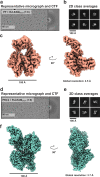
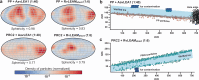
Air-water interface (AWI) interactions during cryo-electron microscopy (cryo-EM) sample preparation cause significant sample loss, hindering structural biology research. Organisms like nematodes and tardigrades produce Late Embryogenesis Abundant (LEA) proteins to withstand desiccation stress. Here we show that these LEA proteins, when used as additives during plunge freezing, effectively mitigate AWI damage to fragile multi-subunit molecular samples. The resulting high-resolution cryo-EM maps are comparable to or better than those obtained using existing AWI damage mitigation methods. Cryogenic electron tomography reveals that particles are localized at specific interfaces, suggesting LEA proteins form a barrier at the AWI. This interaction may explain the observed sample-dependent preferred orientation of particles. LEA proteins offer a simple, cost-effective, and adaptable approach for cryo-EM structural biologists to overcome AWI-related sample damage, potentially revitalizing challenging projects and advancing the field of structural biology.
© 2024. The Author(s).
Conflict of interest statement
A provisional patent has been filed by C.L. for this technology with the Wisconsin Alumni Research Foundation (WARF). The remaining authors declare no competing interests.
Figures

Update of
-
Small LEA proteins as an effective air-water interface protectant for fragile samples during cryo-EM grid plunge freezing.bioRxiv [Preprint]. 2024 Feb 11:2024.02.06.579238. doi: 10.1101/2024.02.06.579238. bioRxiv. 2024. Update in: Nat Commun. 2024 Sep 4;15(1):7705. doi: 10.1038/s41467-024-52091-1 PMID: 38370693 Free PMC article. Updated. Preprint.
Similar articles
-
Small LEA proteins as an effective air-water interface protectant for fragile samples during cryo-EM grid plunge freezing.bioRxiv [Preprint]. 2024 Feb 11:2024.02.06.579238. doi: 10.1101/2024.02.06.579238. bioRxiv. 2024. Update in: Nat Commun. 2024 Sep 4;15(1):7705. doi: 10.1038/s41467-024-52091-1 PMID: 38370693 Free PMC article. Updated. Preprint.
-
Effect of charge on protein preferred orientation at the air-water interface in cryo-electron microscopy.J Struct Biol. 2021 Dec;213(4):107783. doi: 10.1016/j.jsb.2021.107783. Epub 2021 Aug 25. J Struct Biol. 2021. PMID: 34454014
-
Better Cryo-EM Specimen Preparation: How to Deal with the Air-Water Interface?J Mol Biol. 2023 May 1;435(9):167926. doi: 10.1016/j.jmb.2022.167926. Epub 2022 Dec 20. J Mol Biol. 2023. PMID: 36563741 Review.
-
Reducing effects of particle adsorption to the air-water interface in cryo-EM.Nat Methods. 2018 Oct;15(10):793-795. doi: 10.1038/s41592-018-0139-3. Epub 2018 Sep 24. Nat Methods. 2018. PMID: 30250056 Free PMC article.
-
Graphene in cryo-EM specimen optimization.Curr Opin Struct Biol. 2024 Jun;86:102823. doi: 10.1016/j.sbi.2024.102823. Epub 2024 Apr 29. Curr Opin Struct Biol. 2024. PMID: 38688075 Review.
References
MeSH terms
Substances
Grants and funding
- DP2GM150023/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- DP2 GM150023/GM/NIGMS NIH HHS/United States
- R00 GM131023/GM/NIGMS NIH HHS/United States
- R00GM131023/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- T32GM130550/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources


