Sphingosine-1-Phosphate Receptor 4 links neutrophils and early local inflammation to lymphocyte recruitment into the draining lymph node to facilitate robust germinal center formation
- PMID: 39188715
- PMCID: PMC11345157
- DOI: 10.3389/fimmu.2024.1427509
Sphingosine-1-Phosphate Receptor 4 links neutrophils and early local inflammation to lymphocyte recruitment into the draining lymph node to facilitate robust germinal center formation
Abstract
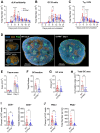
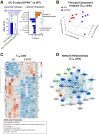
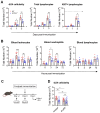
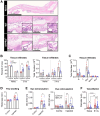
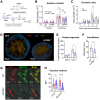
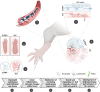
The successful development of germinal centers (GC) relies heavily on innate mechanisms to amplify the initial inflammatory cascade. In addition to their role in antigen presentation, innate cells are essential for the redirection of circulating lymphocytes toward the draining lymph node (dLN) to maximize antigen surveillance. Sphingosine-1-Phosphate (S1P) and its receptors (S1PR1-5) affect various aspects of immunity; however, the role of S1PR4 in regulating an immune response is not well understood. Here we use a footpad model of localized TH1 inflammation to carefully monitor changes in leukocyte populations within the blood, the immunized tissue, and the dLN. Within hours of immunization, neutrophils failed to adequately mobilize and infiltrate into the footpad tissue of S1PR4-/- mice, thereby diminishing the local vascular changes thought to be necessary for redirecting circulating cells toward the inflamed region. Neutrophil depletion with anti-Ly6G antibodies significantly reduced early tissue edema as well as the redirection and initial accumulation of naïve lymphocytes in dLN of WT mice, while the effects were less prominent or absent in S1PR4-/- dLN. Adoptive transfer experiments further demonstrated that the lymphocyte homing deficiencies in vivo were not intrinsic to the donor S1PR4-/- lymphocytes, but were instead attributed to differences within the S1PR4-deficient host. Reduced cell recruitment in S1PR4-/- mice would seed the dLN with fewer antigen-respondent lymphocytes and indeed, dLN hypertrophy at the peak of the immune response was severely diminished, with attenuated GC and activation pathways in these mice. Histological examination of the S1PR4-/- dLN also revealed an underdeveloped vascular network with reduced expression of the leukocyte tethering ligand, PNAd, within high endothelial venule regions, suggesting inadequate growth of the dLN meant to support a robust GC response. Thus, our study reveals that S1PR4 may link early immune modulation by neutrophils to the initial recruitment of circulating lymphocytes and downstream expansion and maturation of the dLN, thereby contributing to optimal GC development during an adaptive response.
Keywords: Sphingosine-1-phosphate receptor 4; germinal center reactions; inflammation; innate cells; lymph node hypertrophy; neutrophils.
Copyright © 2024 Luker, Wukitch, Kulinski, Ganesan, Kabat, Lack, Frischmeyer-Guerrerio, Metcalfe and Olivera.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sphingosine-1-phosphate receptor agonism impairs the efficiency of the local immune response by altering trafficking of naive and antigen-activated CD4+ T cells.J Immunol. 2003 Apr 1;170(7):3662-70. doi: 10.4049/jimmunol.170.7.3662. J Immunol. 2003. PMID: 12646631
-
Chikungunya virus impairs draining lymph node function by inhibiting HEV-mediated lymphocyte recruitment.JCI Insight. 2018 Jul 12;3(13):e121100. doi: 10.1172/jci.insight.121100. JCI Insight. 2018. PMID: 29997290 Free PMC article.
-
Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes.J Immunol. 2014 Aug 15;193(4):1966-74. doi: 10.4049/jimmunol.1301791. Epub 2014 Jul 11. J Immunol. 2014. PMID: 25015824
-
Beyond Immune Cell Migration: The Emerging Role of the Sphingosine-1-phosphate Receptor S1PR4 as a Modulator of Innate Immune Cell Activation.Mediators Inflamm. 2017;2017:6059203. doi: 10.1155/2017/6059203. Epub 2017 Aug 7. Mediators Inflamm. 2017. PMID: 28848247 Free PMC article. Review.
-
The impact of sphingosine-1-phosphate receptor modulators on COVID-19 and SARS-CoV-2 vaccination.Mult Scler Relat Disord. 2023 Jan;69:104425. doi: 10.1016/j.msard.2022.104425. Epub 2022 Nov 22. Mult Scler Relat Disord. 2023. PMID: 36470168 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


