The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration?
- PMID: 39126061
- PMCID: PMC11313076
- DOI: 10.3390/ijms25158490
The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration?
Abstract
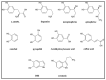
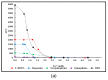
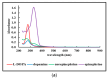
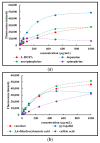
Expanding on earlier observations, we show that many melanin materials, in vitro synthesized from a wide range of precursors, can be fractionated into a dark-colored precipitate and a near-colorless, dispersible fraction. The dispersible fractions exhibited absorbance in the UVA and UVB range of the electromagnetic spectrum, but none in the visible range. In addition, fluorescent properties were associated with all dispersible fractions obtained. FT-IR spectroscopic analyses were performed to compare both types of fractions. Overall, it appears that some of the properties associated with melanin (UV absorbance, fluorescence) may not necessarily reside in the dark-colored portion of melanin, but in a colorless fraction of the material. It remains to be seen whether any of these in vitro observations have any relevance in vivo. However, we raise the possibility that the presence of a colorless fraction within melanin materials and their associated properties may have received inadequate attention. Given the important association between melanin, UV protection, and skin cancer, it is worthwhile to consider this additional aspect of melanin chemistry.
Keywords: FT-IR spectroscopy; UV-Vis spectroscopy; catecholamines; catechols; melanin; serotonin.
Conflict of interest statement
The authors declare conflicts of interest.
Figures




Similar articles
-
Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources.Molecules. 2020 Mar 27;25(7):1537. doi: 10.3390/molecules25071537. Molecules. 2020. PMID: 32230973 Free PMC article. Review.
-
Characterization and photoprotective potentiality of lime dwelling Pseudomonas mediated melanin as sunscreen agent against UV-B radiations.J Photochem Photobiol B. 2021 Mar;216:112126. doi: 10.1016/j.jphotobiol.2021.112126. Epub 2021 Jan 20. J Photochem Photobiol B. 2021. PMID: 33516151
-
The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin.Pigment Cell Melanoma Res. 2011 Feb;24(1):136-47. doi: 10.1111/j.1755-148X.2010.00764.x. Epub 2010 Oct 6. Pigment Cell Melanoma Res. 2011. PMID: 20979596 Free PMC article.
-
New noninvasive approach assessing in vivo sun protection factor (SPF) using diffuse reflectance spectroscopy (DRS) and in vitro transmission.Photodermatol Photoimmunol Photomed. 2014 Aug;30(4):202-11. doi: 10.1111/phpp.12105. Epub 2014 Feb 19. Photodermatol Photoimmunol Photomed. 2014. PMID: 24417335
-
Interactions of Melanin with Electromagnetic Radiation: From Fundamentals to Applications.Chem Rev. 2024 Jun 12;124(11):7165-7213. doi: 10.1021/acs.chemrev.3c00858. Epub 2024 May 17. Chem Rev. 2024. PMID: 38758918 Review.
References
-
- Solano F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014;2014:498276. doi: 10.1155/2014/498276. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


