Phylogenetics, Molecular Species Delimitation and Geometric Morphometrics of All Reddish-Brown Species in the Genus Neotriplax Lewis, 1887 (Coleoptera: Erotylidae: Tritomini)
- PMID: 39057241
- PMCID: PMC11277550
- DOI: 10.3390/insects15070508
Phylogenetics, Molecular Species Delimitation and Geometric Morphometrics of All Reddish-Brown Species in the Genus Neotriplax Lewis, 1887 (Coleoptera: Erotylidae: Tritomini)
Abstract
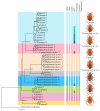
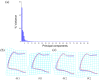
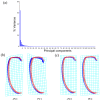
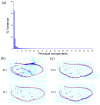
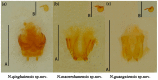
To date, five species of reddish-brown Neotriplax have been described, but their highly similar body color and other phenotypic traits make accurate taxonomy challenging. To clarify species-level taxonomy and validate potential new species, the cytochrome oxidase subunit I (COI) was used for phylogenetic analysis and the geometric morphometrics of elytron, pronotum, and hind wing were employed to distinguish all reddish-brown Neotriplax species. Phylogenetic results using maximum likelihood and Bayesian analyses of COI sequences aligned well with the current taxonomy of the Neotriplax species group. Significant K2P divergences, with no overlap between intra- and interspecific genetic distances, were obtained in Neotriplax species. The automatic barcode gap discovery (ABGD), assemble species by automatic partitioning (ASAP), and generalized mixed Yule coalescent (GMYC) approaches concurred, dividing the similar species into eight molecular operational taxonomic units (MOTUs). Geometric morphometric analysis using pronotum, elytron, hind wing shape and wing vein patterns also validated the classification of all eight species. By integrating these analytical approaches with morphological evidence, we successfully delineated the reddish-brown species of Neotriplax into eight species with three new species: N. qinghaiensis sp. nov., N. maoershanensis sp. nov., and N. guangxiensis sp. nov. Furthermore, we documented the first record of N. lewisii in China. This study underscores the utility of an integrative taxonomy approach in species delimitation within Neotriplax and serves as a reference for the taxonomic revision of other morphologically challenging beetles through integrative taxonomy.
Keywords: DNA barcoding; geometric morphometrics; phylogeny; species delimitation; systematics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











Similar articles
-
DNA barcoding of the genus Gampsocleis (Orthoptera, Tettigoniidae) from China.Arch Insect Biochem Physiol. 2024 Jan;115(1):e22070. doi: 10.1002/arch.22070. Arch Insect Biochem Physiol. 2024. PMID: 38288484
-
Delimiting Species with Single-Locus DNA Sequences.Methods Mol Biol. 2024;2744:53-76. doi: 10.1007/978-1-0716-3581-0_3. Methods Mol Biol. 2024. PMID: 38683311
-
Species delimitation and taxonomic revision of Oxyopes (Araneae: Oxyopidae) of Taiwan, with description of two new species.Zootaxa. 2021 Feb 11;4927(1):zootaxa.4927.1.4. doi: 10.11646/zootaxa.4927.1.4. Zootaxa. 2021. PMID: 33756720
-
To be or not to be… Integrative taxonomy and species delimitation in the daddy long-legs spiders of the genus Physocyclus (Araneae, Pholcidae) using DNA barcoding and morphology.Zookeys. 2022 Dec 12;1135:93-118. doi: 10.3897/zookeys.1135.94628. eCollection 2022. Zookeys. 2022. PMID: 36761795 Free PMC article.
-
Multiple species delimitation approaches with COI barcodes poorly fit each other and morphospecies - An integrative taxonomy case of Sri Lankan Sericini chafers (Coleoptera: Scarabaeidae).Ecol Evol. 2022 May 19;12(5):e8942. doi: 10.1002/ece3.8942. eCollection 2022 May. Ecol Evol. 2022. PMID: 35600695 Free PMC article.
References
-
- McHugh J.V. Description of immature stages for Megischyrus (Erotylidae: Triplacinae) and a review of literature on larval Erotylidae. Ann. Zool. 2001;51:113–124.
-
- Wegrzynowicz P. Morphology, phylogeny and classification of the family Erotylidae based on adult characters (Coleoptera: Cucujoidea) Genus. 2002;13:435–504.
-
- Sato T. Effects of photoperiod and temperature on development and larval diapause of Dacne picta (Coleoptera: Erotylidae) Appl. Entomol. Zool. 2003;38:117–123. doi: 10.1303/aez.2003.117. - DOI
-
- Lewis G. A list of fifty Erotylidae from Japan, including thirty-five new species and four new genera. Ann. Mag. Nat. Hist. 1887;5:53–73. doi: 10.1080/00222938709460010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


