The use of modafinil for the treatment of fatigue in multiple sclerosis: A systematic review and meta-analysis of controlled clinical trials
- PMID: 38988104
- PMCID: PMC11237168
- DOI: 10.1002/brb3.3623
The use of modafinil for the treatment of fatigue in multiple sclerosis: A systematic review and meta-analysis of controlled clinical trials
Abstract
Introduction: Multiple sclerosis (MS) is a debilitating neurological condition affecting nearly one million people across the United States. Among the most prominent symptoms of the condition are excessive fatigue and daytime sleepiness. Numerous clinical trials have investigated the efficacy of modafinil in addressing fatigue among these patients.
Objective: The objective of the present study is to assess the safety and efficacy of modafinil for the treatment of fatigue in MS.
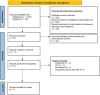
Methodology: An electronic search of PUBMED, ScienceDirect, and Cochrane Central was conducted for articles published from inception to December 2023 using search terms such as "modafinil," "fatigue," and "MS."
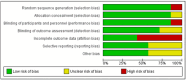
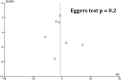
Results: Seven studies were included in our analysis. Modafinil leads to a meaningful reduction in fatigue when compared with placebo, as measured by Modified Fatigue Impact Scale [mean difference (MD) = -4.42 [-8.01, -.84]; I2 = 45%; p = .02] and Epworth Sleepiness Scale [MD = -.87 [-1.64, -.10]; I2 = 0%; p = .03]. Modafinil also demonstrated a greater risk of precipitating adverse events (e.g., insomnia, gastrointestinal symptoms) when compared with placebo [RR = 1.30 [1.03, 1.66]; I2 = 0%; p = .03]. In quality-of-life assessments, modafinil was associated with overall improvement in well-being [standardized mean difference = .18 [.01, .35]; I2 = 56%; p = .04].
Conclusion: The data indicates that modafinil confers a therapeutic benefit when treating fatigue in patients with MS and improves overall quality of life; however, there is a risk of precipitating adverse events. Ultimately, higher quality of evidence may be required to better inform clinical management.
Keywords: MFIS; adverse events; fatigue; modafinil; multiple sclerosis; quality of life.
© 2024 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflicts of interest.
Figures






Similar articles
-
Comparative effectiveness of cognitive behavioural therapy, modafinil, and their combination for treating fatigue in multiple sclerosis (COMBO-MS): a randomised, statistician-blinded, parallel-arm trial.Lancet Neurol. 2024 Nov;23(11):1108-1118. doi: 10.1016/S1474-4422(24)00354-5. Lancet Neurol. 2024. PMID: 39424559 Clinical Trial.
-
Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: a randomised, placebo-controlled, crossover, double-blind trial.Lancet Neurol. 2021 Jan;20(1):38-48. doi: 10.1016/S1474-4422(20)30354-9. Epub 2020 Nov 23. Lancet Neurol. 2021. PMID: 33242419 Free PMC article. Clinical Trial.
-
Meta-analysis of the efficacy of modafinil versus placebo in the treatment of multiple sclerosis fatigue.Mult Scler Relat Disord. 2018 Jan;19:85-89. doi: 10.1016/j.msard.2017.10.011. Epub 2017 Oct 18. Mult Scler Relat Disord. 2018. PMID: 29175676
-
Pharmacological interventions for sleepiness and sleep disturbances caused by shift work.Cochrane Database Syst Rev. 2014 Aug 12;2014(8):CD009776. doi: 10.1002/14651858.CD009776.pub2. Cochrane Database Syst Rev. 2014. PMID: 25113164 Free PMC article. Review.
-
Corticosteroids for the management of cancer-related fatigue in adults with advanced cancer.Cochrane Database Syst Rev. 2023 Jan 23;1(1):CD013782. doi: 10.1002/14651858.CD013782.pub2. Cochrane Database Syst Rev. 2023. PMID: 36688471 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical


