Acetylene and Ethylene Adsorption during Floating Fe Catalyst Formation at the Onset of Carbon Nanotube Growth and the Effect of Sulfur Poisoning: a DFT Study
- PMID: 38986139
- PMCID: PMC11270998
- DOI: 10.1021/acs.inorgchem.4c01830
Acetylene and Ethylene Adsorption during Floating Fe Catalyst Formation at the Onset of Carbon Nanotube Growth and the Effect of Sulfur Poisoning: a DFT Study
Abstract
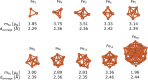
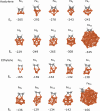
Here, we investigated the adsorption of acetylene and ethylene on iron clusters and nanoparticles, which is a crucial aspect in the nascent phase of carbon nanotube growth by floating catalyst chemical vapor deposition (FCCVD). The effect of sulfur on adsorption was also studied due to its indispensable role in the process and its commonly known impact on metal catalyst poisoning. We performed systematic density functional theory (DFT) computations, considering numerous adsorption configurations and iron particles of various sizes (Fen, n = 3-10, 13, 55). We found that acetylene binds significantly more strongly than ethylene and prefers different adsorption sites. The presence of sulfur decreased the adsorption strength only in the immediate proximity of the adsorbate, suggesting that the effect of sulfur is mainly of steric origin while electronic effects play only a minor role. Higher sulfur coverage of the catalyst surface significantly weakened the binding of acetylene or ethylene. To further investigate this interaction, Bader's atoms in molecules (AIM) analysis and charge density difference (CDD) were used, which showed electron transfer from iron clusters or nanoparticles to the adsorbate molecules. The charge transfer exhibited a decreasing trend as sulfur coverage increased. These results can also contribute to the understanding of other iron-based catalytic processes involving hydrocarbons and sulfur, such as the Fischer-Tropsch synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
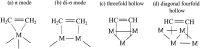


Similar articles
-
The promoter role of sulfur in carbon nanotube growth.Dalton Trans. 2022 Jun 21;51(24):9256-9264. doi: 10.1039/d2dt00355d. Dalton Trans. 2022. PMID: 35667372
-
Structure of Supported and Unsupported Catalytic Rh Nanoparticles: Effects on Nucleation of Single-Walled Carbon Nanotubes.Langmuir. 2017 Oct 24;33(42):11109-11119. doi: 10.1021/acs.langmuir.7b01513. Epub 2017 Jul 28. Langmuir. 2017. PMID: 28709379
-
A DFT study of the adsorption and dissociation of CO on sulfur-precovered Fe100.J Phys Chem B. 2006 Jul 20;110(28):13897-904. doi: 10.1021/jp055979v. J Phys Chem B. 2006. PMID: 16836339
-
Acetylene hydratase: a non-redox enzyme with tungsten and iron-sulfur centers at the active site.J Biol Inorg Chem. 2016 Mar;21(1):29-38. doi: 10.1007/s00775-015-1330-y. Epub 2016 Jan 20. J Biol Inorg Chem. 2016. PMID: 26790879 Review.
-
Recent Developments in Single-Walled Carbon Nanotube Thin Films Fabricated by Dry Floating Catalyst Chemical Vapor Deposition.Top Curr Chem (Cham). 2017 Nov 27;375(6):90. doi: 10.1007/s41061-017-0178-8. Top Curr Chem (Cham). 2017. PMID: 29181596 Review.
References
LinkOut - more resources
Full Text Sources


