Engineering bionanoreactor in bacteria for efficient hydrogen production
- PMID: 38985767
- PMCID: PMC11260135
- DOI: 10.1073/pnas.2404958121
Engineering bionanoreactor in bacteria for efficient hydrogen production
Abstract
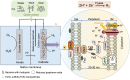
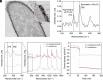
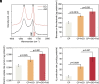
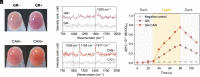
Hydrogen production through water splitting is a vital strategy for renewable and sustainable clean energy. In this study, we developed an approach integrating nanomaterial engineering and synthetic biology to establish a bionanoreactor system for efficient hydrogen production. The periplasmic space (20 to 30 nm) of an electroactive bacterium, Shewanella oneidensis MR-1, was engineered to serve as a bionanoreactor to enhance the interaction between electrons and protons, catalyzed by hydrogenases for hydrogen generation. To optimize electron transfer, we used the microbially reduced graphene oxide (rGO) to coat the electrode, which improved the electron transfer from the electrode to the cells. Native MtrCAB protein complex on S. oneidensis and self-assembled iron sulfide (FeS) nanoparticles acted in tandem to facilitate electron transfer from an electrode to the periplasm. To enhance proton transport, S. oneidensis MR-1 was engineered to express Gloeobacter rhodopsin (GR) and the light-harvesting antenna canthaxanthin. This led to efficient proton pumping when exposed to light, resulting in a 35.6% increase in the rate of hydrogen production. The overexpression of native [FeFe]-hydrogenase further improved the hydrogen production rate by 56.8%. The bionanoreactor engineered in S. oneidensis MR-1 achieved a hydrogen yield of 80.4 μmol/mg protein/day with a Faraday efficiency of 80% at a potential of -0.75 V. This periplasmic bionanoreactor combines the strengths of both nanomaterial and biological components, providing an efficient approach for microbial electrosynthesis.
Keywords: Gloeobacter rhodopsin; H2 production; Shewanella oneidensis MR-1; bionanoreactor; nanomaterials.
Conflict of interest statement
Competing interests statement:W.T., I.P.T., and W.E.H. have filed a provisional patent application with the UK Patent Office related to this work.
Figures
Similar articles
-
Hydrogen production driven by formate oxidation in Shewanella oneidensis MR-1.Appl Microbiol Biotechnol. 2020 Jun;104(12):5579-5591. doi: 10.1007/s00253-020-10608-w. Epub 2020 Apr 17. Appl Microbiol Biotechnol. 2020. PMID: 32303818
-
Divergent Nrf Family Proteins and MtrCAB Homologs Facilitate Extracellular Electron Transfer in Aeromonas hydrophila.Appl Environ Microbiol. 2018 Nov 15;84(23):e02134-18. doi: 10.1128/AEM.02134-18. Print 2018 Dec 1. Appl Environ Microbiol. 2018. PMID: 30266730 Free PMC article.
-
Biological Self-Assembled Transmembrane Electron Conduits for High-Efficiency Ammonia Production in Microbial Electrosynthesis.Environ Sci Technol. 2024 Apr 30;58(17):7457-7468. doi: 10.1021/acs.est.3c10897. Epub 2024 Apr 20. Environ Sci Technol. 2024. PMID: 38642050
-
[Advances in electrochemically active biofilm of Shewanella oneidensis MR-1].Sheng Wu Gong Cheng Xue Bao. 2023 Mar 25;39(3):881-897. doi: 10.13345/j.cjb.220468. Sheng Wu Gong Cheng Xue Bao. 2023. PMID: 36994560 Review. Chinese.
-
Review of cathodic electroactive bacteria: Species, properties, applications and electron transfer mechanisms.Sci Total Environ. 2024 Oct 10;946:174332. doi: 10.1016/j.scitotenv.2024.174332. Epub 2024 Jun 29. Sci Total Environ. 2024. PMID: 38950630 Review.
References
-
- Gernaat D. E. H. J., et al. , Climate change impacts on renewable energy supply. Nat. Clim. Change 11, 119–125 (2021).
-
- Yue M., et al. , Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 146, 111180 (2021).
-
- Turner J. A., Sustainable hydrogen production. Science 305, 972–974 (2004). - PubMed
-
- Wang M., Chen L., Sun L., Recent progress in electrochemical hydrogen production with earth-abundant metal complexes as catalysts. Energy Environ. Sci. 5, 6763–6778 (2012).
-
- International Energy Agency, Global Hydrogen Review 2023 (International Energy Agency, Paris, France, 2023). https://www.iea.org/reports/global-hydrogen-review-2023.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


