Subregion-specific transcriptomic profiling of rat brain reveals sex-distinct gene expression impacted by adolescent stress
- PMID: 38977070
- PMCID: PMC11444371
- DOI: 10.1016/j.neuroscience.2024.07.002
Subregion-specific transcriptomic profiling of rat brain reveals sex-distinct gene expression impacted by adolescent stress
Abstract
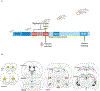
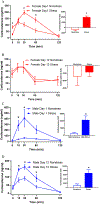
Stress during adolescence clearly impacts brain development and function. Sex differences in adolescent stress-induced or exacerbated emotional and metabolic vulnerabilities could be due to sex-distinct gene expression in hypothalamic, limbic, and prefrontal brain regions. However, adolescent stress-induced whole-genome expression changes in key subregions of these brain regions were unclear. In this study, female and male adolescent Sprague Dawley rats received one-hour restraint stress daily from postnatal day (PD) 32 to PD44. Corticosterone levels, body weights, food intake, body composition, and circulating adiposity and sex hormones were measured. On PD44, brain and blood samples were collected. Using RNA-sequencing, sex-specific differences in stress-induced differentially expressed (DE) genes were identified in subregions of the hypothalamus, limbic system, and prefrontal cortex. Canonical pathways reflected well-known sex-distinct maladies and diseases, substantiating the therapeutic potential of the DE genes found in the current study. Thus, we proposed specific sex distinct, adolescent stress-induced transcriptional changes found in the current study as examples of the molecular bases for sex differences witnessed in stress induced or exacerbated emotional and metabolic disorders. Future behavioral studies and single-cell studies are warranted to test the implications of the DE genes identified in this study in sex-distinct stress-induced susceptibilities.
Keywords: Amygdala; Chronic restraint stress; Hippocampus; Hypothalamus; Orbitofrontal cortex; Sex-specific differences.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Variations in Phase and Amplitude of Rhythmic Clock Gene Expression across Prefrontal Cortex, Hippocampus, Amygdala, and Hypothalamic Paraventricular and Suprachiasmatic Nuclei of Male and Female Rats.J Biol Rhythms. 2015 Oct;30(5):417-36. doi: 10.1177/0748730415598608. Epub 2015 Aug 13. J Biol Rhythms. 2015. PMID: 26271538 Free PMC article.
-
Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats.Hippocampus. 2011 Nov;21(11):1216-27. doi: 10.1002/hipo.20829. Epub 2010 Jul 21. Hippocampus. 2011. PMID: 20665592
-
Acute stress imposed during adolescence yields heightened anxiety in Sprague Dawley rats that persists into adulthood: Sex differences and potential involvement of the Medial Amygdala.Brain Res. 2019 Nov 15;1723:146392. doi: 10.1016/j.brainres.2019.146392. Epub 2019 Aug 22. Brain Res. 2019. PMID: 31446016 Free PMC article.
-
Sexually-dimorphic alterations in cannabinoid receptor density depend upon prenatal/early postnatal history.Neurotoxicol Teratol. 2016 Nov-Dec;58:31-39. doi: 10.1016/j.ntt.2016.09.004. Epub 2016 Sep 12. Neurotoxicol Teratol. 2016. PMID: 27634313 Free PMC article. Review.
-
Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive- and anxiety-like symptoms.Brain Struct Funct. 2017 Jan;222(1):1-20. doi: 10.1007/s00429-016-1218-9. Epub 2016 Mar 31. Brain Struct Funct. 2017. PMID: 27033097 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


