The dual role of sirtuins in cancer: biological functions and implications
- PMID: 38947884
- PMCID: PMC11211395
- DOI: 10.3389/fonc.2024.1384928
The dual role of sirtuins in cancer: biological functions and implications
Abstract
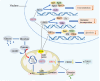
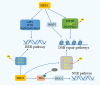
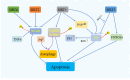
Sirtuins are pivotal in orchestrating numerous cellular pathways, critically influencing cell metabolism, DNA repair, aging processes, and oxidative stress. In recent years, the involvement of sirtuins in tumor biology has garnered substantial attention, with a growing body of evidence underscoring their regulatory roles in various aberrant cellular processes within tumor environments. This article delves into the sirtuin family and its biological functions, shedding light on their dual roles-either as promoters or inhibitors-in various cancers including oral, breast, hepatocellular, lung, and gastric cancers. It further explores potential anti-tumor agents targeting sirtuins, unraveling the complex interplay between sirtuins, miRNAs, and chemotherapeutic drugs. The dual roles of sirtuins in cancer biology reflect the complexity of targeting these enzymes but also highlight the immense therapeutic potential. These advancements hold significant promise for enhancing clinical outcomes, marking a pivotal step forward in the ongoing battle against cancer.
Keywords: cancer therapy; histone deacetylase; metabolic functions; sirtuins; tumor.
Copyright © 2024 Yu, Li, Song, Zhang, Wang, Wang, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Sirtuins and cognition: implications for learning and memory in neurological disorders.Front Physiol. 2022 Jul 22;13:908689. doi: 10.3389/fphys.2022.908689. eCollection 2022. Front Physiol. 2022. PMID: 35936890 Free PMC article. Review.
-
Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility.Hum Reprod Update. 2018 May 1;24(3):267-289. doi: 10.1093/humupd/dmy003. Hum Reprod Update. 2018. PMID: 29447380 Review.
-
Implications of altered sirtuins in metabolic regulation and oral cancer.PeerJ. 2023 Feb 14;11:e14752. doi: 10.7717/peerj.14752. eCollection 2023. PeerJ. 2023. PMID: 36815979 Free PMC article. Review.
-
The emerging and diverse roles of sirtuins in cancer: a clinical perspective.Onco Targets Ther. 2013 Oct 8;6:1399-416. doi: 10.2147/OTT.S37750. Onco Targets Ther. 2013. PMID: 24133372 Free PMC article. Review.
-
Role of sirtuins in ischemia-reperfusion injury.World J Gastroenterol. 2013;19(43):7594-602. doi: 10.3748/wjg.v19.i43.7594. World J Gastroenterol. 2013. PMID: 24616566 Free PMC article. Review.
Cited by
-
Sirtuin 5 (SIRT5) Suppresses Tumor Growth by Regulating Mitochondrial Metabolism and Synaptic Remodeling in Gliomas.Int J Mol Sci. 2024 Aug 22;25(16):9125. doi: 10.3390/ijms25169125. Int J Mol Sci. 2024. PMID: 39201811 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources


