Establishment of dry-chemistry-based reference intervals of routine liver function tests for the adult population of Gandaki Province, Nepal
- PMID: 38722987
- PMCID: PMC11081272
- DOI: 10.1371/journal.pgph.0001865
Establishment of dry-chemistry-based reference intervals of routine liver function tests for the adult population of Gandaki Province, Nepal
Abstract
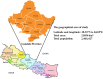
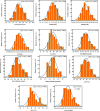
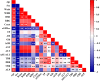
Every clinical laboratory should ideally establish its own population-specific reference intervals (RIs) to promote precision and evidence-based medicine. However, clinical laboratories in Nepal find it easier to follow external RIs than establish their own, leading to a lack of RIs specific to the local population. This study thus aimed to establish RIs of routine LFTs for the adult population of Gandaki Province, Nepal, and compare them with the current RIs used by our laboratory. We established the dry-chemistry-based reference intervals of 11 common LFT parameters for the adult population of Gandaki Province, Nepal using the direct priori-based method. The combined and sex-specific 95% double-sided RIs of total protein, albumin, globulin, A/G ratio, bilirubin, aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), AST/ALT ratio, and alkaline phosphatase (ALP) were established using non-parametric percentile method. The new RIs were also compared with the currently used RIs that were adopted from the reagent kit inserts. The newly established RIs for each LFT were: Total proteins: 68.0-69.0g/L, albumin: 39.0-52.0g/L; globulin: 27.0-42.0g/L; A/G ratio: 1.1-1.8; total bilirubin: 5.13-25.65μmol/L (0.30-1.50mg/dl); unconjugated bilirubin: 1.71-17.10μmol/L (0.10-1.00mg/dl); conjugated bilirubin: 0.00-10.26 μmol/L (0.00-0.60mg/dl); AST: 20.0-43.2U/L; ALT: 11.0-53.0 U/L; AST/ALT ratio: 0.7-2.1; ALP: 42.0-135.4U/L. The RIs of albumin, globulin, A/G ratio, AST, ALT, and AST/ALT ratio differed significantly (p < 0.05) between males and females. Moreover, calculated out-of-range values showed that up to 4-40% of apparently healthy adults were classified as having abnormal test results based on current RIs. The newly established RIs fulfil the need for population and platform-specific RIs for the adult population of Gandaki Province of Nepal and bring more conformity and accuracy in interpreting the LFT results, diagnosis of hepatobiliary diseases, clinical decision-making, monitoring the success of therapy and future liver specific biomedical researches within the Gandaki Province of Nepal.
Copyright: © 2024 Sharma et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Establishment of dry chemistry based reference intervals of renal function test parameters for the adult population of Kaski District, Nepal.BMC Nephrol. 2021 Oct 6;22(1):331. doi: 10.1186/s12882-021-02542-4. BMC Nephrol. 2021. PMID: 34615479 Free PMC article.
-
Reference intervals for total bilirubin, ALT, AST and creatinine in healthy Chinese elderly.Med Sci Monit. 2014 Oct 1;20:1778-82. doi: 10.12659/MSM.892148. Med Sci Monit. 2014. PMID: 25272068 Free PMC article.
-
Reference intervals of routine clinical chemistry parameters among apparently healthy young adults in Amhara National Regional State, Ethiopia.PLoS One. 2018 Aug 2;13(8):e0201782. doi: 10.1371/journal.pone.0201782. eCollection 2018. PLoS One. 2018. PMID: 30071088 Free PMC article.
-
Distinguishing reference intervals and clinical decision limits - A review by the IFCC Committee on Reference Intervals and Decision Limits.Crit Rev Clin Lab Sci. 2018 Sep;55(6):420-431. doi: 10.1080/10408363.2018.1482256. Epub 2018 Jul 26. Crit Rev Clin Lab Sci. 2018. PMID: 30047297 Review.
-
The Role and Limitations of the Reference Interval Within Clinical Chemistry and Its Reliability for Disease Detection.Br J Biomed Sci. 2024 Feb 28;81:12339. doi: 10.3389/bjbs.2024.12339. eCollection 2024. Br J Biomed Sci. 2024. PMID: 38481978 Free PMC article. Review.
References
-
- Mijač D, Krstić MN, Marković AP, Popović DD, Krstić JM, Milosavljević T. Abnormal Liver Blood Tests: Primary Care Approach. Dig Dis. 2022;40(2):215–22. - PubMed
-
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59(8):2223–30. - PubMed
-
- Gary L. Horowitz Sousan Altaie, PhD, James C. Boyd MD et al.. MD. EP28-A3c: Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline—Third Edition. Clin Lab Stand Inst. 2010;(October).
Grants and funding
LinkOut - more resources
Full Text Sources

