Bortezomib promotes the TRAIL-mediated killing of resistant rhabdomyosarcoma by ErbB2/Her2-targeted CAR-NK-92 cells via DR5 upregulation
- PMID: 38706988
- PMCID: PMC11067460
- DOI: 10.1016/j.omton.2024.200802
Bortezomib promotes the TRAIL-mediated killing of resistant rhabdomyosarcoma by ErbB2/Her2-targeted CAR-NK-92 cells via DR5 upregulation
Abstract
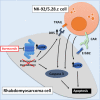
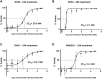
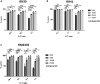
Treatment resistance and immune escape are hallmarks of metastatic rhabdomyosarcoma (RMS), underscoring the urgent medical need for therapeutic agents against this disease entity as a key challenge in pediatric oncology. Chimeric antigen receptor (CAR)-based immunotherapies, such as the ErbB2 (Her2)-CAR-engineered natural killer (NK) cell line NK-92/5.28.z, provide antitumor cytotoxicity primarily through CAR-mediated cytotoxic granule release and thereafter-even in cases with low surface antigen expression or tumor escape-by triggering intrinsic NK cell-mediated apoptosis induction via additional ligand/receptors. In this study, we showed that bortezomib increased susceptibility toward apoptosis in clinically relevant RMS cell lines RH30 and RH41, and patient-derived RMS tumor organoid RMS335, by upregulation of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor DR5 in these metastatic, relapsed/refractory (r/r) RMS tumors. Subsequent administration of NK-92/5.28.z cells significantly enhanced antitumor activity in vitro. Applying recombinant TRAIL instead of NK-92/5.28.z cells confirmed that the synergistic antitumor effects of the combination treatment were mediated via TRAIL. Western blot analyses indicated that the combination treatment with bortezomib and NK-92/5.28.z cells increased apoptosis by interacting with the nuclear factor κB, JNK, and caspase pathways. Overall, bortezomib pretreatment can sensitize r/r RMS tumors to CAR- and, by upregulating DR5, TRAIL-mediated cytotoxicity of NK-92/5.28.z cells.
Keywords: MT: Regular Issue; NK cells; TRAIL; bortezomib; chimeric antigen receptor; immunotherapy; rhabdomyosarcoma.
© 2024 The Author(s).
Conflict of interest statement
J.-H.K. has advisory roles for Bluebird Bio, Novartis, Roche, and Jazz Pharmaceuticals. T.T. and W.S.W. are named as inventors on patents and patent applications related to the therapeutic agent used in this study, owned by their respective academic institutions.
Figures




Similar articles
-
ERBB2-CAR-Engineered Cytokine-Induced Killer Cells Exhibit Both CAR-Mediated and Innate Immunity Against High-Risk Rhabdomyosarcoma.Front Immunol. 2020 Oct 19;11:581468. doi: 10.3389/fimmu.2020.581468. eCollection 2020. Front Immunol. 2020. PMID: 33193388 Free PMC article.
-
ErbB2 (HER2)-CAR-NK-92 cells for enhanced immunotherapy of metastatic fusion-driven alveolar rhabdomyosarcoma.Front Immunol. 2023 Aug 18;14:1228894. doi: 10.3389/fimmu.2023.1228894. eCollection 2023. Front Immunol. 2023. PMID: 37662907 Free PMC article.
-
CAR-mediated targeting of NK cells overcomes tumor immune escape caused by ICAM-1 downregulation.J Immunother Cancer. 2024 Feb 27;12(2):e008155. doi: 10.1136/jitc-2023-008155. J Immunother Cancer. 2024. PMID: 38417916 Free PMC article.
-
Retargeting of NK-92 Cells against High-Risk Rhabdomyosarcomas by Means of an ERBB2 (HER2/Neu)-Specific Chimeric Antigen Receptor.Cancers (Basel). 2021 Mar 22;13(6):1443. doi: 10.3390/cancers13061443. Cancers (Basel). 2021. PMID: 33809981 Free PMC article.
-
CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy.Front Immunol. 2019 Nov 14;10:2683. doi: 10.3389/fimmu.2019.02683. eCollection 2019. Front Immunol. 2019. PMID: 31798595 Free PMC article. Review.
References
-
- Varlet P., Bouffet E., Casanova M., Giangaspero F., Antonelli M., Hargrave D., Ladenstein R., Pearson A., Hawkins C., König F.B., et al. Comprehensive analysis of the ErbB receptor family in pediatric nervous system tumors and rhabdomyosarcoma, Pediatr. Blood Cancer. 2022;69 doi: 10.1002/pbc.29316. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

