Signaling controversy and future therapeutical perspectives of targeting sphingolipid network in cancer immune editing and resistance to tumor necrosis factor-α immunotherapy
- PMID: 38698424
- PMCID: PMC11064425
- DOI: 10.1186/s12964-024-01626-6
Signaling controversy and future therapeutical perspectives of targeting sphingolipid network in cancer immune editing and resistance to tumor necrosis factor-α immunotherapy
Abstract
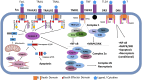
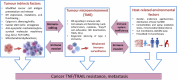
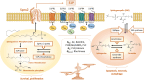
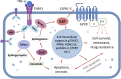
Anticancer immune surveillance and immunotherapies trigger activation of cytotoxic cytokine signaling, including tumor necrosis factor-α (TNF-α) and TNF-related apoptosis-inducing ligand (TRAIL) pathways. The pro-inflammatory cytokine TNF-α may be secreted by stromal cells, tumor-associated macrophages, and by cancer cells, indicating a prominent role in the tumor microenvironment (TME). However, tumors manage to adapt, escape immune surveillance, and ultimately develop resistance to the cytotoxic effects of TNF-α. The mechanisms by which cancer cells evade host immunity is a central topic of current cancer research. Resistance to TNF-α is mediated by diverse molecular mechanisms, such as mutation or downregulation of TNF/TRAIL receptors, as well as activation of anti-apoptotic enzymes and transcription factors. TNF-α signaling is also mediated by sphingosine kinases (SphK1 and SphK2), which are responsible for synthesis of the growth-stimulating phospholipid, sphingosine-1-phosphate (S1P). Multiple studies have demonstrated the crucial role of S1P and its transmembrane receptors (S1PR) in both the regulation of inflammatory responses and progression of cancer. Considering that the SphK/S1P/S1PR axis mediates cancer resistance, this sphingolipid signaling pathway is of mechanistic significance when considering immunotherapy-resistant malignancies. However, the exact mechanism by which sphingolipids contribute to the evasion of immune surveillance and abrogation of TNF-α-induced apoptosis remains largely unclear. This study reviews mechanisms of TNF-α-resistance in cancer cells, with emphasis on the pro-survival and immunomodulatory effects of sphingolipids. Inhibition of SphK/S1P-linked pro-survival branch may facilitate reactivation of the pro-apoptotic TNF superfamily effects, although the role of SphK/S1P inhibitors in the regulation of the TME and lymphocyte trafficking should be thoroughly assessed in future studies.
Keywords: Apoptosis; Cancer drug resistance; Immunotherapy; Sphingolipids; Sphingosine kinase; Sphingosine-1-phosphate; Tumor necrosis factor-α.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target.Pharmacol Ther. 2020 Mar;207:107464. doi: 10.1016/j.pharmthera.2019.107464. Epub 2019 Dec 18. Pharmacol Ther. 2020. PMID: 31863815 Review.
-
Sphingolipids as mediators of inflammation and novel therapeutic target in inflammatory bowel disease.Adv Protein Chem Struct Biol. 2020;120:123-158. doi: 10.1016/bs.apcsb.2019.11.003. Epub 2020 Jan 8. Adv Protein Chem Struct Biol. 2020. PMID: 32085881 Review.
-
Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming.Int J Mol Sci. 2018 Jan 31;19(2):420. doi: 10.3390/ijms19020420. Int J Mol Sci. 2018. PMID: 29385066 Free PMC article. Review.
-
Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha.J Biol Chem. 2005 Jul 29;280(30):27879-87. doi: 10.1074/jbc.M503002200. Epub 2005 Jun 9. J Biol Chem. 2005. PMID: 15946935
-
Sphingolipid metabolism in cancer signalling and therapy.Nat Rev Cancer. 2018 Jan;18(1):33-50. doi: 10.1038/nrc.2017.96. Epub 2017 Nov 17. Nat Rev Cancer. 2018. PMID: 29147025 Free PMC article. Review.
Cited by
-
RANK-RANKL-OPG Axis in MASLD: Current Evidence Linking Bone and Liver Diseases and Future Perspectives.Int J Mol Sci. 2024 Aug 24;25(17):9193. doi: 10.3390/ijms25179193. Int J Mol Sci. 2024. PMID: 39273141 Free PMC article. Review.
References
-
- Kearney CJ, Vervoort SJ, Hogg SJ, Ramsbottom KM, Freeman AJ, Lalaoui N, Pijpers L, Michie J, Brown KK, Knight DA, Sutton V, Beavis PA, Voskoboinik I, Darcy PK, Silke J, Trapani JA, Johnstone RW, Oliaro J. Tumor immune evasion arises through loss of TNF sensitivity. Sci Immunol. 2018;3(23):eaar3451. doi: 10.1126/sciimmunol.aar3451. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 075-15-2022-1128/Ministry of Science and Higher Education of the Russian Federation at FRC Kazan Scientific Center
- 075-15-2022-1128/Ministry of Science and Higher Education of the Russian Federation at FRC Kazan Scientific Center
- 222300420534/National Natural Science Foundation of Henan, China
- 222300420534/National Natural Science Foundation of Henan, China
LinkOut - more resources
Full Text Sources


