Complement networks in gene-edited pig xenotransplantation: enhancing transplant success and addressing organ shortage
- PMID: 38566098
- PMCID: PMC10986007
- DOI: 10.1186/s12967-024-05136-4
Complement networks in gene-edited pig xenotransplantation: enhancing transplant success and addressing organ shortage
Abstract
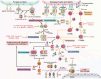
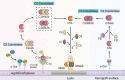
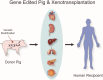
The shortage of organs for transplantation emphasizes the urgent need for alternative solutions. Xenotransplantation has emerged as a promising option due to the greater availability of donor organs. However, significant hurdles such as hyperacute rejection and organ ischemia-reperfusion injury pose major challenges, largely orchestrated by the complement system, and activated immune responses. The complement system, a pivotal component of innate immunity, acts as a natural barrier for xenotransplantation. To address the challenges of immune rejection, gene-edited pigs have become a focal point, aiming to shield donor organs from human immune responses and enhance the overall success of xenotransplantation. This comprehensive review aims to illuminate strategies for regulating complement networks to optimize the efficacy of gene-edited pig xenotransplantation. We begin by exploring the impact of the complement system on the effectiveness of xenotransplantation. Subsequently, we delve into the evaluation of key complement regulators specific to gene-edited pigs. To further understand the status of xenotransplantation, we discuss preclinical studies that utilize gene-edited pigs as a viable source of organs. These investigations provide valuable insights into the feasibility and potential success of xenotransplantation, offering a bridge between scientific advancements and clinical application.
Keywords: Clinical trials; Complement systems; Genetically modified pigs; Xenotransplantation; α-1,3-galactosyltransferase gene-knockout.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no competing interests related to this review.
Figures

Similar articles
-
Cutting edge of genetically modified pigs targeting complement activation for xenotransplantation.Front Immunol. 2024 Apr 4;15:1383936. doi: 10.3389/fimmu.2024.1383936. eCollection 2024. Front Immunol. 2024. PMID: 38638432 Free PMC article. Review.
-
Xenotransplantation: Current Status in Preclinical Research.Front Immunol. 2020 Jan 23;10:3060. doi: 10.3389/fimmu.2019.03060. eCollection 2019. Front Immunol. 2020. PMID: 32038617 Free PMC article. Review.
-
Xenotransplantation of solid organs in the pig-to-primate model.Transpl Immunol. 2009 Jun;21(2):87-92. doi: 10.1016/j.trim.2008.10.005. Epub 2008 Oct 26. Transpl Immunol. 2009. PMID: 18955143 Review.
-
Coagulation dysregulation as a barrier to xenotransplantation in the primate.Transpl Immunol. 2009 Jun;21(2):75-80. doi: 10.1016/j.trim.2008.10.008. Epub 2008 Nov 10. Transpl Immunol. 2009. PMID: 19000927 Free PMC article. Review.
-
The role of genetically engineered pigs in xenotransplantation research.J Pathol. 2016 Jan;238(2):288-99. doi: 10.1002/path.4635. Epub 2015 Oct 7. J Pathol. 2016. PMID: 26365762 Free PMC article. Review.
References
-
- <https://www.organdonor.gov/learn/organ-donation-statistics> (Health Resources & Services Administration).
Publication types
MeSH terms
Grants and funding
- 8200062054/National Natural Science Foundation of China
- 81771723/National Natural Science Foundation of China
- 2020M683295/China Postdoctoral Science Foundation
- ZYGX2021YGLH024/medical and industrial integration funded project of University of Electronic Science and Technology of China
- 2022YFS0157/Department of Science and Technology of Sichuan Province
LinkOut - more resources
Full Text Sources


